SL01
SOME NOVEL METHODS OF INVESTIGATION OF TRANSPORT PARAMETERS OF GASES AND VAPORS IN POLYMER MEMBRANES
KAREL FRIESS, VLADIMÍR HYNEK, MILAN ŠÍPEK
Department of Physical Chemistry, Institute of Chemical Technology, Technická 5, Prague 6, 166 28
Czech Republic, tel.:+420/220444029, fax: +420/220444333, e-mail: karel.friess@vscht.cz
1. Introduction
The detailed knowledge of structure/permeability relations of polymers and information of their interactions with penetrants as well play an important role in industrial applications of polymer materials like protective coating, packaging of food and beverages or in membrane separation processes (MSP). In past decades, the MSP as the low-cost energy-saving techniques constituted a hopefully alternative to the classical technologies, especially in highly needed removal of volatile organic compounds (VOCs) from water or air [1-4]. Just the shortcomings and limitations of VOCs removal methods compel to searching of new polymer materials with better parameters (combining of rigid "hard" segments interspaced with flexible "soft" segments, imbedding of various-scale-fillers into polymer matrix etc) leading to the increasing of system performance (high selectivity, long lifetime, suitable mechanical properties) [3-6]. Therefore, many experimental studies of permeation, diffusion or sorption of gases and vapors in polymers are performed to obtain information about transport parameters and decide possible polymer utilisation.
2. Theory
For description of transport of gases and vapors through non-porous polymer membranes the solution-diffusion model [1] is frequently used. Based on this model, the mass transport process of one component through non-porous polymer membranes can be described as a sequence of consecutive processes of sorption of the components on the feed side of the membrane surface, activated diffusion through membrane and desorption of the components on the permeate side of the membrane. An association of diffusion with sorption (solubility) of permeation rate together enabled to simply express otherwise a complex process of mass transport caused by external driving forces (temperature, pressure and concentration gradients) and effects of interactions between polymer material and penetrating components [2-4].
P=DS (1)
where P is the permeability coefficient, D is the diffusion coefficient and S the sorption coefficient.
Based on the 1st Fick´s law, the density of permeation flux J of the measured gas or vapor penetrating through membrane in the direction of x coordinate can be expressed as [4,9,13,14]:
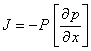 , (2)
, (2)
where (¶p/¶x) is the pressure gradient. Description of mass transport in ideal ternary system (1 and 2 penetrating components, 3 polymer) constitutes more complicated problem [6-8]. For case of isothermal transport of binary mixture in form of molar fluxes J through finite membrane with pressure gradient as a driving force can be expresed as [6-9]
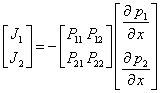 (3)
(3)
where P11 and P22 are own permeability coefficients of components 1 and 2 and P12 and P21 are off-diagonal coefficients which represent influence of one component to permeation of the other one.
Other ways of description of multicomponent transport enable extended Fick's first law [8,9], linear irreversible thermodynamics (LNRT)[10] or Maxwell-Stefan [11,12] model.
3. Determination of permeability coefficient by differential permeation method
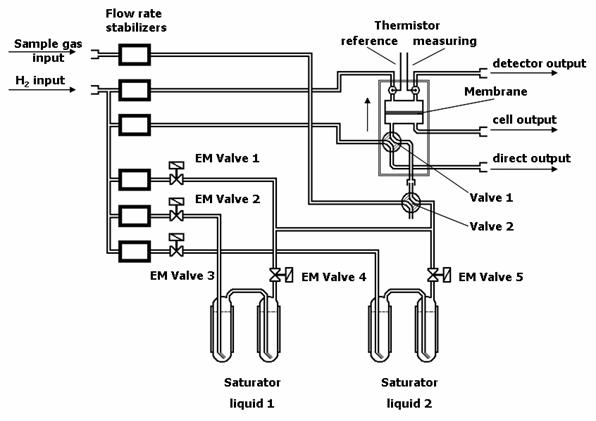
One of the methods how to determine permeability coefficients of gases and vapors through polymer membranes is differential permeation method [4,14,15]. Detailed description of the differential flow permeameter (Fig. 1), experimental set up and calculation of permeability coefficients is mentioned elsewhere [15,16]. The sample of flat polymer membrane is fixed in a permeation cell of permeameter and puts into contact with a mixture of gases or vapors with hydrogen (carrier gas) at constant pressure in order to obtain a step concentration. The penetrating gas or vapors from the downstream (feed) side of the membrane to upstream side are from upper department of cell carried away by a stream of pure hydrogen. Changes of thermal conductivity of hydrogen (due to vapors permeation) are monitored by a pair of the thermal conductivity detectors built into the Wheatstone resistance bridge. The reference thermistor is in permanent contact with pure carrier gas while the measuring one is in contact with mixture of permeant and hydrogen. A stable voltage signal from the resistance bridge is obtained until reaching of steady-state. Hence, the values of the steady-state voltage signal (NS)can be used for expressing the permeability coefficients of measured gas or vapors [13,14,16] as
![]() , (4)
, (4)
where k is an apparatus constant (determined by calibration), l is a membrane thickness, v flow rate of the mixture, A is a membrane area, T is the temperature of experiment, T0 is the standard temperature 273.15 K, p0 is the standard pressure 101.325 kPa, pV is the partial pressure of vapors in carrier gas (for gases pV = pB) and pB is a barometric pressure. Maximal error of determination of P values reached 5-10 % in dependence of kinds of polymers.
From permeation experimental data also the diffusion coefficient should be calculated. Calculations are based on solution of the second Fick's law under initial and boundary conditions which fit the experimental set-up.
Connecting of differential flow permeameter to gas chromatograph (GC) with mass spectrometer (MS) make possible to follow and determine molar flux densities of gases and vapor penetrating through membrane during whole experiment (fast loop sampling). Sample of gaseous mixture from upper department of the permeation cell can be taken directly to the loop (volume of 1 ml) and transfer to the GC+MS. The values of molar flux densities of gases or vapors can be calculated from knowledge of peak areas, calibration constants for each compounds, membrane area, volume of sampling loop and flow rate through loop. From such experiments the mutual influence of penetrating components during mass transfer through membrane could be investigated.
4. Estimation of permeability coefficients from sorption data
The most of sorption experiments are performed gravimetrically (microbalances, Kahn´s balances or spiral balances). The major difference between permeation and sorption methods consist in fact that sorption measurements run till sorption equilibrium while the permeation ones till steady-state. From sorption data can be calculated the sorption coefficients and the diffusion coefficients and the permeability coefficients can be estimated by the stationary (steady) diffusion theory.
The stationary (steady) diffusion theory for sorption [4,14,16] is based on assumption of establishing a hypothetical steady state between the surface and the center of the membrane (half of membrane thickness l/2), where the flux J of component i and the concentration gradient (¶ci /¶z) from the 1st Fick´s law can be replaced by their time-period averages, as
![]() , (5)
, (5)
where DI is an integral diffusion coefficient which in narrow concentration interval represents mean value of differential diffusion coefficient. The advantage of this theory consists in possibility to evaluate the permeability coefficients from sorption data. For sorption data the kinetic equation of the first order due to the difference of vapor activities inside and outside of the membrane for measured sorption data has been applied. The relationship for absorbed mass of vapors m in time t is given by equation (6)
![]() , (6)
, (6)
where m¥ is the mass uptake at infinite time (in sorption equilibrium), C is a velocity constant of sorption experiment (obtained by data fitting), and J is the density of mass flux, which is defined as
![]() . (7)
. (7)
In equation (7) the symbol A represents a surface area on one side of the membrane (i.e. one half of membrane thickness), joining of equations (6) and (7) leads to
![]() . (8)
. (8)
The time-period average value of flux density can be obtained by integration of equation (5):
![]() . (9)
. (9)
Thus, the permeability coefficient (in units: m mol m-2 s-1 Pa-1) from sorption measurements can be calculated (with respect to total membrane thickness) by equation
![]() , (10)
, (10)
where M is the molecular weight of penetrant, l is the membrane thickness and p is the equilibrium vapor pressure.
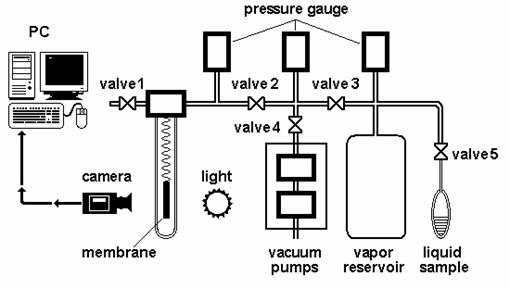
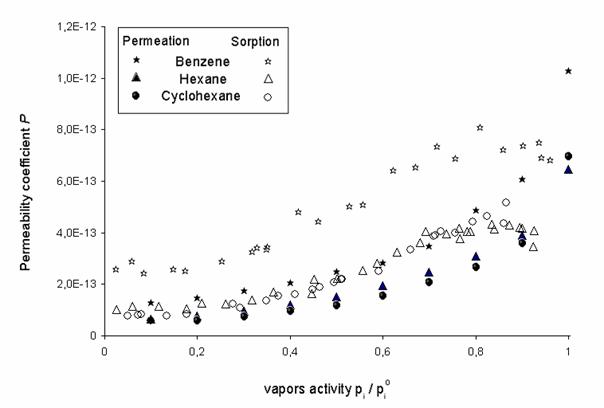
Comparison of values of the permeability coefficients of benzene, hexane and cyclohexane through polymer membrane calculated from permeation data (Eq.4) and from sorption ones (Eq. 10), which were obtained by sorption apparatus equipped with calibrated quartz (McBain´s) spiral balance (Fig.2), are shown on Fig.3 [16]. For experiments the high-pressure, low density Polyethylene BRALEN FB2-30 supplied by Slovnaft, a.s., Slovak Republic, thickness 50±1 mm, the volume fraction of crystalline phase 45.5 %, polymer density at 298.15 K was r = (919 ± 2) kg m-3 [12] was used.
The sorption apparatus on Fig.2 is equipped with calibrated spiral balance (with known sensitivity of uptake mass of compound [mg] over stretching of spiral [mm]). The stripe of sample polymer membrane (weight 0.5 - 2 g) is hanged on a spiral in evacuated glass tube. Stretching of quartz spiral is monitored by the optical system (camera SONY XCD-X710 FireWire with objective SE2514, Sony Corp., Japan, software and installation Neovision s.r.o., Czech Republic) at selected time intervals (0.5 - 100 s) from the beginning of measurement till the equilibrium state. The liquid sample (5-10 ml) is degassed before dosing from glass vessel connected to apparatus. Selected vapors tension (i.e. activity) of each experiment is set by calibrated feed valve and determined by pressure gauge. The vapors reservoir serves for pressure equalization. Before each experiment the apparatus is evacuated to pressure c. 0.001Pa by rotary and turbo molecular pumps. The average error of mass determination reaches approximately 25-30 mg.
5. Determination of sorption coefficients from very low penetrant concentrations
The Vapor Phase Calibration (VPC) technique [17] is a simple and effective in studying sorption of VOCs or gases in polymer membranes and enables to determine sorption of vapors at very low vapors concentrations (0.01 - 1 % of vapors pressure at given temperature).
In the VPC method same amount of penetrant, m is injected to a two-phase-system (membrane-air) and to an one-phase system (air). The headspace of two systems is analysed by GC. Advantage of this procedure consist in fact that experiments can run without need of performing a standard calibration in advance because is directly known the difference between sorption in two-phase system in comparison with one-phase system where no sorption takes place.
Equations (11) and (12) represent the mass balance over both systems at equilibrium:
m=mm+mg (11)
CVVV=Cm+CgVg (12)
where mm and mg are the equilibrium masses of penetrant in the membrane phase and the gas phase, respectively (two-phase-system), CV is the analyte's concentration in the one-phase-system and VV, Vg, Vm are volumes of the one-phase-system, headspace and the membrane matrix of the gas-membrane-system, respectively. By this method the solubility coefficient can be calculated as
![]() (13)
(13)
where AV and Ag are peaks areas of penetrant in the one-phase-system and the headspace of gas-membrane system respectively.
REFERENCES
[1] S.C. George, S. Thomas, Transport phenomena through polymeric systems, Prog. Polym. Sci. 26 (2001) 985-1017
[2] J.G. Wijmans, R.W. Baker, The solution-diffusion model: a review, Journal of Membrane Science, 107 (1995) 1-21
[3] Osada Y., Nakagawa T.; Membrane Science and Technology, Marcel Dekker Inc., New York, (1992).
[4] M.H.V. Mulder, Basic Principles of Membrane Technology, Kluwer Academic Publishers, Dordrecht (1998).
[5] T. T. Moore, W. J. Koros, Non-ideal effects in organic-inorganics materials for gas separation membranes, Journal of Molecular Structure, 739 (2005) 87-98
[6] T.K. Sherwood, R.L. Pigford, C.R. Wilke, Mass Transfer, McGraw-Hill, New York (1975).
[7] E.L. Cussler, Diffusion - Mass transfer in fluid systems, Cambridge University Press, Cambridge (1984).
[8] P. Kerkhof, M. Geboers, Analysis and extension of the theory of multicomponent fluid diffusion", Chem. Eng. Sci., Vol. 60, 12 (2005) 3129-3167
[9] H.D. Kamaruddin, W. J. Koros, Some observations about the application of Fick's first law for membrane separation of multicomponent mixtures. J. Memb. Sci 135 (1997) 14-159
[10] B. Baranowski, Non-equilibrium thermodynamics as applied to membrane transport, J. Membr. Sci., 57 (1991) 119-159
[11] R. Krishna, J.A. Wesselingh, The Maxwell-Stefan approach to mass transfer. Chemical Engineering Science, vol 52, 6 (1997) 861-911
[12] P. Izák, L. Bartovská, K. Friess, M. Šípek, P. Uchytil, Description of Binary Liquid Mixtures Transport through Non-porous Membrane by Modified Maxwell-Stefan Equations. J. Membr. Sci., 214 (2003) 293-309
[13] J. Crank, The Mathematic of Diffusion, Clarendon Press, Oxford (1975).
[14] Crank J., Park G. S.; Diffusion in Polymers, Academia Press, London (1968).
[15] V. Hynek, M. Šípek, Chem. Listy, 90 (1996) 938-951
[16] K. Friess, M. Šípek, V. Hynek, P. Sysel, K. Bohatá, P. Izák, Comparison of permeability coefficients of organic vapors through non-porous polymer membranes by two experimental techniques, J. Mem. Sci, 240 (2004) 179-185
[17] I. De Bo, H. Van Langenhove, J. De Keijser, Application of vapour phase calibration method for determination of sorption of gases and VOC in PDMS membranes, J. Mem. Sci. 209 (2002) 39-52
Acknowledgements: This work was supported by the grant of MSM No. 6046137307 of Czech Ministry of Education, Youth and Sports.
|
|
|
Fig. 1. Differential flow permeameter |
|
|
|
Fig.2. The sorption apparatus |
|
|
|
Fig.3. Comparison of permeability coefficients P [mol m-1 Pa-1 s-1] of VOCs through LDPE membrane obtained by sorption and permeation from K. Friess at al, J. Mem. Sci, 240 (2004) 179-185 |