Posters
1 2 3 4 5 6 7 8
P01
NOVEL HYDROPHILIC/HYDROPHOBIC POLYMER BLENDS AS MEMBRANE MATERIALS
Jun Qiu, K.-V. Peinemann
GKSS Forschungszentrum GmbH, 21502 Geesthacht, Germany
Two phase interpenetrating polymeric structures may show unexpected
transport properties, which cannot be predicted easily by the pure
phases. A famous example of a two phase interpenetrating membrane
material is DuPont's Nafion, which consists of a perfluorcarbon matrix
containing a second continuous phase comprised of sulfonated
perfluorocarbon. The hydrophobic perfluorocarbon phase prevents
excessive swelling of the hydrophilic sulfonic acid phase. This leads
to unusual high cation permselectivity. In this lecture preparation
methods and transport properties of new blends of hydrophilic and
hydrophobic polymers are presented. These blends can usually not been
prepared by solution casting, because in most cases no common solvents
exist for the two polymers. We solved this problem by a " Trojan Horse"
approach. The hydrophilic polymer was transferred by a simple chemical
modification into a hydrophobic polymer, which subsequently could be
mixed with a second hydrophobic polymer. After obtaining the
solvent-free blend the chemically modified hydrophobic polymer (the
Trojan Horse) was changed back into the original hydrophilic polymer.
By this method unique blends could be prepared.
Transport properties for gaseous and liquid mixtures will be discussed.
P02
IONOMER CHARACTERIZATION FOR USE IN FUEL CELLS
F. J. Fernández-Carretero,a* V. Compañ,a, E. Riande,b R. Díaz-Calleja,a A. Quijano,c
a Departamento de Termodinámica Aplicada. ETSII. Universidad Politécnica de Valencia. 46020, Valencia. Spain
b Instituto de Ciencia y Tecnología de Polímeros (CSIC), 28006, Madrid. Spain
c Instituto de Tecnología Eléctrica, 46980, Valencia. Spain
*fraferc4@etsii.upv.es
INTRODUCTION
Ionic conductive solid materials are good electrolyte candidates for
fuel cells, electrodialyzers, batteries, sensors, etc. The growing
interest in the development of fuel cells and batteries has prompted a
flourishing research in the preparation of ionic organic solid
conductors based on polymers due to the excellent mechanical properties
and easy processing of these materials. From a practical point of view,
ion-exchange membranes displaying good performance for a variety of
applications should exhibit high ionic selectivity, good ionic
conductance, low permeability to free diffusion of electrolytes and low
electroosmosis, as well as chemical stability, high mechanical
resistance, high flexibility and good dimensional stability at the
working conditions. Moreover, fuel diffusion across membranes should be
severely hindered. Some of these properties are incompatible and
therefore it is necessary to reach a balance among them in order to
design membranes with optimum performance, .
The deleterious permeation effect of perfluorocarbon commercial
membranes together with their still high cost and negative
environmental effects have promoted the search for alternatives to
these materials.
MEMBRANE PREPARATION
Semicrystalline polypropylene (Repsol) and EPDM Vistalon 9500
(Exxon Chemical, France), a polyolefinic terpolymer containing 60 wt %
of ethylene, 29 wt % of propylene, and 11 wt % of
5-ethylidine-2-norbornene, were used. The 5-ethylidine-2-norbornene
units were sulfonated using the procedure developed by Makowski et. al.
In brief, acetic anhydride and concentrated sulphuric acid were slowly
added to a solution of EPDM in n-hexane under vigorous stirring. The
temperature of the solution was not allowed to exceed 0 ºC and the
reaction was stopped after 30 min by adding alcohol to the reaction
medium. The acidic form of the sulfonated polymer was further separated
by adding deionized water to the reaction medium and flashing off the
hydrocarbon solvents. The polymer was washed several times with water
until neutral pH was obtained and dried at 100 ºC.
Two blends of sulfonated EPDM and polypropylene (PP) containing 0.10
(wt/wt) (ICTP1) and 0.20 (wt/wt) (ICTP2) of the latter component
respectively, were prepared in a Brabender torque rheometer using a
thermoplastic mixing chamber (type W60) of 75 cm3 of capacity, keeping the temperature in the preheating step at 453 K. The rotor speed was set at 55 rev min-1
and once the torque was constant the materials was sheared in the
chamber for about 10 min more to ensure good blending homogenization. A
small amount of butyl phthalate was used as plasticizer in the blending
process.
Membranes were prepared from the sulfonated blends by compression at
200 bars in a Collins Hydraulic press for about 20 min. During
compression the samples were kept at 200 ºC for 10 min and then cooled
down to room temperature without removing the pressure
A commercial Nafion 117 membrane manufactured by DuPont was used as
a referent to check the electrochemical performance of the membranes
prepared from sulfonated EPDM-polypropylene blends.
CHARACTERIZATION
Water uptake
Dry sulfonated membranes in the acidic (R-SO3H) form were
weighed and further immersed in distilled water. From time to time the
membranes were removed from water, superficially dried by gently
pressing between filter paper and weighed. This operation was repeated
until a constant weight was reached. From the weights of the dried and
wet membranes the water uptake was obtained, and the pertinent results
are shown in Table 1.
Ion-exchange capacity
The membranes were equilibrated in a 2 M HCl solution overnight. The
acidic membranes were further washed several times with distilled water
and then equilibrated with a 2 M sodium chloride solution. The protons
delivered after the ion-exchange reaction R-SO3H + Na+ →R-SO3Na + H+
took place were titrated with a 0.01 M sodium hydroxide solution. The
values of the ion-exchange capacity of the membranes used in this study
are collected in Table 1.
|
Membrane
|
Weight of the wet membrane (g)
|
Weight of the dry membrane (g)
|
Exchange capacity H+ (mol/kg of dry membrane)
|
Water uptake (%)
|
H+ (mol/kg of water in the membrane)
|
|
ICTP1
|
0.1170
|
0.0816
|
2.45
|
43.38
|
5.65
|
|
ICTP2
|
0.0939
|
0.0761
|
1.27
|
23.35
|
5.43
|
|
Nafion 117
|
0.1417
|
0.1180
|
1.35
|
20.09
|
6.70
|
Table 1 Water uptake and ionic exchange capacity
It can be seen that water uptake decreases in ICTP2 due to the
percent of EPDM is lower than in ICTP1 so there are less hydrophilic
groups (5-ethylidine-2-norbornene units). In this way, ionic exchange
capacity of ICTP2 is also low than ICTP1. Both membranes have suitable
values of the IEC for its use as ionic exchange membranes.
Transport Number
Transport numbers were determined according to the irreversible
processes thermodynamics to characterize the transport properties of
the membranes, , , , .
Experimental electrochemical potentials were measured in a cell with the following configuration:
Ag | AgCl | HCl solution (cL)| membrane | HCl solution (cR) | AgCl | Ag.
cL is fixed, 0,01M, while cR varies from 0,001 to 1.
The cell employed is made of Pyrex glass and the membrane was tightly campled between two compartments of 150 cm3
capacity. The electrodes were dipped into the cell and connected to a
multimeter HP 3441. The electromotive force was recorded with a PC that
took measures every 5 s. During the measurements, the cell solutions
were stirred to minimize polarization effects.
Experiments have been repeated changing HCl solutions for NaCl to
study the membrane behaviour when the size of the ion raises (H+ vs. Na+).
Results are shown in table 2, where one can see that near 1 transport numbers are obtained when working with H+ as a result of good ionic exchange properties. When using Na+ the increase in ion size results in a decrease of the transport numbers.
|
Steady state
|
|
|
medio
|
|
ICTP1 H+
|
0,9602
|
|
ICTP2 H+
|
0,9701
|
|
Nafion H+
|
0,9721
|
|
ICTP1 Na+
|
0,6830
|
|
ICTP2 Na+
|
0,7187
|
|
Nafion Na+
|
0,6751
|
Table 2 Membranes transport number
Electrical analysis
Proton conductivity was measured using impedance spectroscopy using
a dielectric spectrometer Novocontrol with Alpha analyzer and Quatro
temperature control. Samples were measured sandwiched in the liquid
sample cell BDS 1308 in distilled water to simulate 100% RH. Frequency range was from 10-2 to 106
Hz in isothermal conditions with amplitude of 1V.Conductivity has been
obtained from intersection with the real impedance axis in the high
frequency side of Nyquist and, using a second method, from the Bode
plots, obtaining conductivity when phase angle trends to 0 in the high
frequency area.
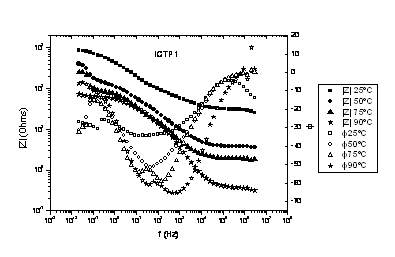
Fig. 1 ICTP1 Bode plot
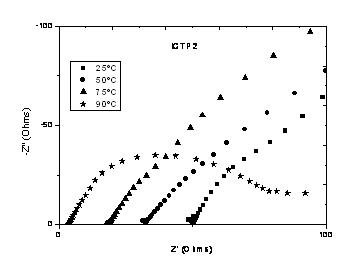
Fig. 2 ICTP2 Nyquist Plot
Conductivities are shown in table 3,
|
|
ICTP1
|
ICTP2
|
Nafion 117
|
|
T(ºC)
|
(S/cm)
|
(S/cm)
|
(S/cm)
|
|
25
|
9,84E-04
|
7,25E-04
|
5,73E-03
|
|
50
|
8,17E-03
|
1,12E-03
|
8,98E-03
|
|
75
|
1,60E-02
|
2,01E-03
|
1,30E-02
|
|
90
|
8,35E-02
|
1,03E-02
|
1,16E-02
|
Table 3 Conductivities of the membranes
Conductivity is better in ICTP1; this was expected because ICTP1 has
the highest ionic exchange capacity. And there's an increase in the
conductivity when the temperature raises form 75 to 90ºC in the EPDM
membranes, while there's no raise in Nafion one's.
CONCLUSIONS
The comparison of the electrochemical properties of the ICTP1 and
ICTP2 membranes expressed in terms of the conductivity and proton
transport number with those of Nafion, suggests that the ICTP1 membrane
exhibits similar performance as the Nafion membrane.
However, to conclude whether the ICTP membranes could be used in
fuel cells would require to investigate their chemical and mechanical
stability at high temperatures.
ACKNOWLEDGEMENTS
This work was supported by the AVCT ( Generalitat Valenciana), CAM
(Comunidad Autónoma de Madrid) and the DGCYT through Grants
Grupos03/030, IMCITA2005/31, GR/MAT/0723/2004, and MAT2002-04042-C0-1,
respectively.
REFERENCES
Lakshminarayanaiah, N. Transport phenomena in membranes, Academic Press, London, 1969
Riande, E. Transport
phenomena in ion-exchange membranes, In Physics of electrolytes, Vol.
I, J. Hladik, ed., Academic Press, London, 1972; Chapter XI
Makowski, H.S., Plains, S., Lundberg, R.D., Block, J. U.S. Patent 1980, 4,184,988
J. Garrido, V. Compañ, V.M. Aguililla, S. Mafé. Electrochem. Acta 35 (1990) 4 705-709
J. Garrido, V. Compañ, M.L. López, D.G. Millar. J. Phys. Chem. B 101 (1997) 29 5740-5746
V. Compañ, T.S. Sorensen, A. Andrio, L. López, J. de Abajo. J. Memb. Sci. 123 (1997) 293-302
J. Garrido, V. Compañ, M.L. López. Curr. Top. Electrochem. 4 (1997) 115-126
J. Garrido, V. Compañ, M.L. López. Phys. Rev. E 64 (2001) 016122
A. Mokrini, M.A. Huneault, J. Pow. Sourc. 154 (2006) 51-58
R. Mohr, V. Kudela, J. Schauer, K. Richau, Deslination 147 (2002) 191-196
P 03
THERMAL BEHAVIOR AND MORPHOLOGY OF BIODEGRADABLE P(3HB)-BASED MEMBRANES
K. Wesslera, A.P.T. Pezzinb, S.H. Pezzinb
aCenter for Technological Sciences, Santa
Catarina State University - UDESC, Campus Universitário s/n, CP 631,
89223-100 Joinville-SC, Brazil (pezzin@joinville.udesc.br)
bUNIVILLE, CP 246, 89201-972 Joinville-SC, Brazil
Summary. The miscibility behavior and the morphology of
blends of poly(3-hydroxybutyrate), P(3HB), and polycaprolactone triol,
PCL-T, obtained by casting, were studied by differential scanning
calorimetry, thermogravimetric analysis, X-ray diffraction, infrared
spectroscopy and scanning electron microscopy. It was observed that
higher PCL-T ratios results in transparent, porous and flexible films.
Polyhydroxyalkanoates (PHAs) are thermoplastic polyesters made from
bacterial fermentation using renewable resources. The polymers are
produced as an intracellular storage polymer of carbon and energy under
various nutritional and environmental conditions (1). In the past two
decades, PHAs have been the focus of extensive research considering
their potential application as biocompatible and biodegradable
thermoplastics, due to their hydrolyzability in the human body as well
as in natural circumstances (2). The polymers of PHAs family more
extensively studied are poly(3-hydroxybutyrate), P(3HB) and the
copolymer poly(3-hydroxybutyrate)-co-(3-hydroxyvalerate) (P(3HB-co-3HV)).
P(3HB) can in principle be used in many applications, however, since
P(3HB) is highly crystalline and forms large spherulites and also has a
relatively high glass transition temperature in comparison with
polypropylene, polyethylene, etc., the material itself is regarded as
unacceptable brittle. Furthermore, P(3HB) suffers from an economic
disadvantage and limited processing temperatures. These drawbacks have
restricted the widespread application of P(3HB) (3). On the other hand,
poly(caprolactone - triol) (PCL-T) is a semi-crystalline polymer with
melting point around 30şC and glass transition temperature around
-68şC, being potentially interesting to be used in biomaterials (4).
Thus, it seems that PCL-T can be a good choice to plasticize P(3HB-co-3HV), maintaining its biodegradability and bioreabsorption properties.
Aiming the development of new ductile and porous biodegradable materials, blends of P(3HB) and P(3HB-co-3HV)
with polycaprolactone triol (PCL-T) in compositions varying from 100/0
to 70/30 P(3HB)/PCL-T (w/w), were obtained by casting. P(3HB) and P(3HB-co-3HV) used
in this research were kindly supplied by PHB Industrial S.A. and
purified prior to use by dissolution in chloroform and precipitation in
n-hexane. The number average molecular weight of P(3HB) and P(3HB-co-3HV), obtained by GPC, was around 3.0 x 105 g mol-1. PCL-T with 900 g mol-1 was purchased from Aldrich. Membranes
were obtained by casting from 1% chloroform solutions. The solution was
poured in a glass mold and placed in an evaporator system for 48 hours.
The films were completely dried in a vacuum oven at 60°C for 24 hours
and stored in a desiccator. The blends were further analyzed by X-ray
diffraction (XRD), differential scanning calorimetry (DSC),
thermogravimetric analysis (TGA), and scanning electron microscopy
(SEM).
The DSC data for P(3HB) blends are summarized in Table 1. It
was observed that the PCL-T melting temperatures did not change in all
compositions, while the P(3HB) melting temperatures decreases a little
when the PCL-T content is increased. It was also noted that the Tg of
PHB did not vary in function of the PCL-T content. The Tg of
the samples with low plasticizer content are badly defined, however
those from samples with higher PCL-T contents are best defined,
suggesting that the PCL-T addition diminishes the crystallinity of the
sample. These results suggest that the P(3HB)/PCL-T system is
immiscible. Indeed, FTIR analyses did not show evidences of hydrogen
bonds between the polymers. XRD analysis confirmed that the addition of
PCL-T to P(3HB) causes a decrease of ca. 30% on the crystallinity,
independently of the PCL-T content.
Table 1: DSC data (Tm) for PHB/PCL-T blends
|
Blendas P(3HB)/PCL-T
|
Tg PHB (şC)
|
Tm PCL-T (şC)
|
Tm P(3HB) (şC)
|
|
95/05
|
- 2.4
|
22.8
|
171.4
|
|
90/10
|
- 2.7
|
22.8
|
170.9
|
|
85/15
|
- 2.4
|
22.8
|
168.5
|
|
80/20
|
- 2.3
|
23.9
|
169.0
|
|
70/30
|
- 2.3
|
23.1
|
164.0
|
For P(3HB-co-3HV) blends, it was observed (Table 2) that the
glass transition temperatures decreased from 1,66şC to ca. -10şC with
the addition of 15 wt% of PCL-T. Further addition of PCL-T does not
modifies the Tg of P(3HB-co-3HV). It is also noticed that the melting temperatures of P(3HB-co-3HV)
and PCL-T decreases gradually as the PCL-T content is increased. The Tg
of the samples are best defined with higher PCL-T contents, suggesting
that the PCL-T addition decreases the crystallinity of the sample.
These results evidence a partial miscibility between PCL-T in P(3HB-co-3HV).
Table 2. DSC data (Tm) and (Tg) for P(3HB-co-3HV)/PCL-T 900 blends.
|
P(3HB-co-3HV)/
PCL-T
|
Tg (şC)
P(3HB-co-3HV)
|
Tm PCLT (şC)
|
Tc (şC)
P(3HB-co-3HV)
|
Tm (şC)
P(3HB-co-3HV)
|
|
100/0
|
1.66
|
-
|
53.36
|
172.0
|
|
90/10
|
-9.27
|
30.70
|
-
|
170.3
|
|
85/15
|
-10.43
|
20.04
|
53.19
|
168.5
|
|
80/20
|
-10.36
|
25.43
|
46.92
|
165.6
|
|
70/30
|
-10.18
|
24.20
|
50.32
|
164.0
|
Thermal degradation studies were carried out by thermogravimetric
methods, showing that the addition of PCL-T slightly decreases the
onset temperature of the P(3HB) degradation. SEM micrographs
revealed that higher PCL-T ratios result in very porous membranes. It
is also noted that the transparency of the membranes increases with the
PCL-T content, as well as the flexibility. These results suggest that
these materials have great potential to biomedical applications and
packings.
References
[1] P. D'haene, E.E. Remsen, J. Asrar. Macromolecules 32, 5229-5235 (1999).
[2] H. Tsuji, K. Suzuyoshi. Polym. Degrad. Stab. 75, 347-355 (2002).
[3] W. Chen, D.J. David, W.J. MacKnight, F.E. Karasz. Polymer 42, 8407-8414 (2001).
[4] 3. H.R. Lin, C.J. Kuo, Y.J. Lin. J.Cell.Plast. 39, 101 (2003).
P04
GRAFTING OF POLY(STYRENE-CO-MALEIC ANHYDRIDE) WITH POLY(ETHYLENE GLYCOL) FOR MEMBRANE PREPARATION
Diógenes Rojas, Peter F.W. Simón, Volker Abetz
GKSS Forschungzentrum Gesthaacht GmbH, Max Planck Str. 1, Gesthacht, D-21502 Germany
Diogenes.rojas/gkss.de
In the last year the use of copolymer membranes has emerged as a new
alternative and it has interested too many researchers. The main focus
of this area is to find the structure/property relation in polymers
materials and correlation with gas separation membranes properties. In
this way, the use of self-assembly polymer material which lead up to
obtain well-ordered structures due to the ease of accessing complex
structures with small features sizes (1-4).
The aims are to investigate the relation between the morphology and
the overall composition of Poly(Ethylene Glycol) (PEG) /
Poly(Styrene-co-Maleic Anhydride) (SMA) and their functionality, as
well as, to study the effect of the morphology on the membrane
properties (permeability, selectivity and diffusion). In our
experiments we use poly(styrene-co-maleic anhydride) and
poly(ethylene glycol). These copolymers are used to prepare the new
copolymers by esterification reaction and then all these products are
characterized by 1H-NMR, DSC, TGA and GPC. The dense
membrane method by casting is used to prepare films and then theses are
analyzed for contact angle, AFM, SEM, and finally are measured their
properties. Our first conclusion shows that due to the esterification
reaction it is possible to link and to increase PEG contents in the
matrix SMA which helps to reduce the glass transition temperature (Tg)
in the films. The contact angle showed that the membranes have more
hydrophilic behavior in comparison to the original matrix. And finally,
the functionality play particular role in the behavior of the membrane
properties.
References
- J.N Chan, G. D. Stucky, D. E. Morse, T.J. Deming, Nature 2000, 403, 289
- G.M. Whitesides. B. Grzybowski, Science 2002,295,2418
- Abetz, V.; Simon, P.W.F. Adv. Pol. Sci. 2005, 189,125
- S. H. Kim, M. J. Misner , T. P. Russell Adv. Mater. 2004, 16, 3 ,226
P05
6F6F AS GAS SEPARATION MEMBRANE. EFFECT OF THE FORMATION TEMPERATURE ON PERMSELECTIVITY
Roberto RecioVázquez1, Laura Palacio1, Pedro Prádanos1, Antonio Hernández1,
Ángel E. Lozano2, Ángel Marcos, José G. de la Campa2, Javier de Abajo2
1 Dpto. Física Aplicada, Universidad de Valladolid, Facultad de Ciencias, Real de Burgos s/n, 47071 Valladolid. Spain
2 Dpto. Química Macromolecular, Instituto de Polímeros, CSIC, Juan de la Cierva 3, 28006 Madrid. Spain
Over the last decades, polymeric membranes have proven to operate
successfully in industrial gas separations. To obtain membranes that
combine high permeability and high selectivity together with thermal
stability, new polymers, high added value materials, so-called
high-performance polymers, were developed like polyimide (PI). Several
aromatic polyimides as 6F6F have shown promising properties when
separating gas mixtures like O2/N2 and CO2/CH4.
In addition, a inverse relationship between permeability and
selectivity is observed, which fit a limit to the development of new
polymer structure that show a landmark in this field.
Nevertheless, membranes used for gas separation at the moment are of
solution-diffusion type polymeric membranes. One of the immediate
challenges facing membrane material design is achieving higher
permselectivity with equal or greater productivity compared to existing
materials. A high selectivity leads to a high purity of products and
allows a reduction in the number of operation steps and thus a cutback
in the needed membrane area. A high permeability involves a high
process velocity or productivity.
In this work, transport properties like permeability and selectivity
of gas separation membranes made from 6FDA-6FpDA with a
N,N-dimethylacetamide (DMAc) as solvent have been measured in a time
lag equipment. It has been shown that an increase in the temperature of
formation of the membrane leads to better selectivity versus
permeability compromise. This has been tested for O2/N2 and CO2/CH4 gas pairs.
An increase in the temperature of formation, to approach the boiling
temperature of the solvent, increases both the glass transition
temperature and the fractional free volume. These factors help to
explain the structural changes that allow simultaneous improvements of
permeability and selectivity.
P06
EFFECT OF FULLERENE ADDITIVES ON PERVAPORATION PROPERTIES OF POLY(PHENYLENE OXIDE) MEMBRANES
A.V. Penkovaa, A.M. Toikkaa, G.A. Polotskayab
a Dep. Chem. Thermodynamics & Kinetics, St.
Petersburg State University, University pr. 26, Petrodvoretz, St.
Petersburg, 198504, Russia (stasya84@nm.ru)
bInstitute of Macromolecular Compounds, Russian Academy of Sciences, St. Petersburg, Bolshoy pr. 31, 199004, Russia
The allotropic form of carbon known as fullerene exhibits unique
physico-chemical properties and can provide a possibility for
modification of polymer membrane. It has been shown that fullerene C60 improves polyphenylene oxide (PPO) transport properties in gas separation [1]. The combination of С60
and PPO gives a donor-acceptor complex that leads to increasing
membrane density and influence on gas transport properties. In the
present work, fullerene-containing PPO membranes are considered in
pervaporation processes. The effect of fullerene additives on PPO
transport properties is studied in the pervaporation of the reacting
mixture: ethanol - acetic acid - water - ethyl acetate in binary,
ternary, and quaternary combination of components. The shifting of the
esterification equilibrium:
C2H5OH + CH3COOH = Н2О + CH3COOC2H5 is one of the ways for maximizing yield of ethyl acetate in hybrid process involving pervaporation.
Homogeneous C60-PPO membranes containing up to 2 %wt C60 (~60 mm thick) were prepared by mixing PPO and C60 solutions in toluene, by casting the solution on a cellophane surface, and by drying at 40оС.
These membranes were studied in pervaporation of mixtures involving
ethanol, acetic acid, ethyl acetate, and water in different
combination. It was shown that permeate is enriched by ethyl acetate in
pervaporation of four-component mixture in equilibrium composition.
Acetic acid does not essentially permeate through all membranes under
study. High selectivity with respect to ethyl acetate was established
in pervaporation of two-component ethyl acetate/water mixtures. The use
of fullerene-containing membranes made it possible to increase
selectivity to 700 for 7% ethyl acetate in water (azeotrope). High
selectivity with respect to ethyl acetate is caused by hydrophobic
properties of our membranes.
Pervaporation properties of membrane depend on interactions between
components of the feed solution and the polymer of the membrane. To
estimate the interaction, equilibrium vapour sorption of ethanol,
acetic acid, water, and ethyl acetate was studied. Fullerene-containing
membranes show a better sorption capacity for ester, acid, and alcohol
than that of PPO membranes. Ethyl acetate exhibits the highest sorption
(the lowest value of χ), then acetic acid and ethanol come.
Water is absolutely inert to polymers under study. These results are in
good agreement with data on pervaporation.
Thus, it was established that fullerene as a component of the С60-PPO composition profoundly affects membrane properties.
Acknowledgement. This work was supported by Russian Foundation for Basic Research (grant 06-03-32493).
Reference
- G.Polotskaya, Yu.Biryulin, Z.Pientka, L.Brozova, M.Bleha,
Transport properties of fullerene - polyphenylene oxide homogeneous
membranes, Fullerenes, Nanotubes, and Carbon Nanostructures, 12(1) (2004) 387-391
polyaniline based responsive membrane
SUDIP RAY, NEIL R. EDMONDS AND ALLAN J. EASTEAL
Polymer Electronics Research Centre, Department of Chemistry, The
University of Auckland, Private Bag 92019, Auckland, New Zealand
(s.ray/auckland.ac.nz)
Responsive or 'smart' polymer materials are functional, structured
systems that show selective response to external (environmental)
conditions, such as temperature, light, pH, stress, strain, electric or
magnetic field. Polyaniline (PANI) has been investigated as a potential
membrane material for gas separation, due to its controllable
electrical conductivity, environmental stability and interesting redox
properties associated with the chain nitrogens. The present work is
designed to fabricate PANI based responsive membranes in different
ways. One of the key problems related to the application of PANI,
however, is its poor processability. The most promising approach to
this problem is the preparation of PANI blends with processable
thermoplastic polymers. Thus, solution and melt blending methods were
applied with polyvinylidene fluoride (PVDF) and/or polymethyl
methacrylate (PMMA) as the blending component(s) or as the support
material(s).
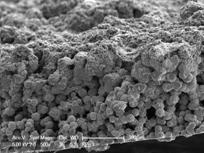 | 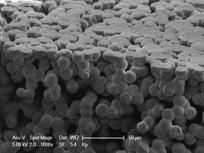 |
|
FIG. 1: SEM images of (a) porous PVDF as the support material and | (b) in-situ polymerized PANI over porous PVDF support.
|



