PC 37
NOVEL COPOLYMERS BASED ON FLUORENE AND THIOPHENE: SYNTHESES, PHOTOPHYSICAL AND ELECTROCHEMICAL PROPERTIES
P. Pavlačková, V. Cimrová, D. Výprachtický, I. Kmínek
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
(pavlackova@imc.cas.cz, cimrova@imc.cas.cz, vyprach@imc.cas.cz)
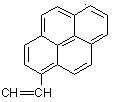
Novel p-conjugated fluorene - thiophene copolymers were synthesized by Suzuki coupling reaction of bis(propane-1,3-diyl)9,9-dihexylfluorene-2,7-bisboronate and 3-substituted (R = 3-methylbutyl, hexyl, 2-pyren-1-ylvinyl) 2,5-dibromothiophenes.
|
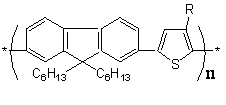 |
FIPT |
F3HP |
FPyT |
|||
|
|
|
Photoluminescence (PL) and electrochemical behavior of these polymers were studied. PL behavior of FPyT polymer was different from that of FIPT, F3HT polymers. Thin films of FIPT and F3HT exhibited an intense green PL emission whereas the PL emission of FPyT thin films was orange. The PL emission spectra in thin films differ from those measured in THF solutions. The red-shift of the PL maximum in thin film compared with that in solution was much larger in FPy thin films than in the two other thin films indicating pronounced aggregation of FPyT in the solid state. All the polymers oxidized and reduced reversibly. Similar values of ionization potential (HOMO level) and electron affinity (LUMO level) were estimated for FIPT and F3HT, but different for FPyT.
We would like to acknowledge the support of the Ministry of Education, Youth and Sports of the Czech Republic (grant No.1M06031) and of the Grant Agency of the Academy of Sciences of the Czech Republic (grant No. IAA4050409).