| a | b | c |
 |
 |
 |
CHARACTERIZATION OF NEW COMPLEXES BASED ON THE INTERACTION OF METALLOPORPHYRINS WITH POLYMER CARRIERS
N.M. Zhunusbekovaa, N.B. Sarovaa, T.K. Jumadilova, E.A. Bekturova
a Laboratory of Physical Chemistry, A.B. Bekturov Institute of Chemical Sciences, Valikhanov Str. 106, Almaty, 050010, Kazakhstan, (znazym@mail.ru, jumadilov@mail.ru)
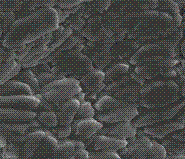
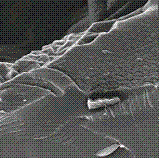
Interaction between hydrogel based on poly(acrylic acid) (PAAc) and metalloporphyrins (PhpMe) in isopropyl alcohol solution leads to the formation of ternary complexes stabilized by coordination bonds. For this purpose porphyrin (phaeophytin-b) was extracted from plant raw materials of Kazakhstan. To obtain the metalloporphyrins the metal ions Al+, Na+, Co2+, Ni2+, Fe3+ were used. In this study the new polymeric materials were prepared by the radical polymerizationof acrylic acid and subsequent reaction with metalloporphyrins. The quantitative determination of interacted PhpMe with PAAc-gel was achieved by UV-vis spectroscopy by analyzing of a liquid over the hydrogel. The greatest value of crosslinking degree of the metalloporphyrins with hydrogel was found for PhpAl and the least for PhpCo. It can be related to inconvenient incorporation of the Co2+ ions to the plane of the porphyrin ligand. The morphological changes of the obtained polymeric materials were observed by scanning electron microscopy (Fig.1). It is obviously shown the influence of incorporated metal ions to morphology of obtained complexes. The potential applications of these materials will be discussed.
|
FIG.1. The morphological changes of hydrogelPAAc with Php (a), PhpNa (b), PhpCo (c).