PC 01
π-CONJUGATE COVALENT LAYERED NETWORKS DERIVED FROM CYANURIC CHLORIDE AND CERTAIN AROMATIC DIAMINES
P. Dallas*1, A.B. Bourlinos*2, Y. Sanakis, D. Petridis, D. Niarchos
NCSR Demokritos, Institute of Materials Science, 15310, Athens, Greece
E-mail: 1dallas@ims.demokritos.gr, 2bourlinos@ims.demokritos.gr
Nucleophilic substitution of the reactive chlorine atoms in cyanuric chloride by bridging 4,4'-bipyridine, benzidin or 1,4 phenylendiamine units in refluxing toluene leads to the formation of a π-conjugate, covalent layered network1. The network is composed of central 1,3,5-triazine units with diamine molecules covalently attached and balanced by the released chloride ions. This results in a two-dimensional layered network as proposed by the XRD patterns, while a radical concentration of 1x1020 spin g-1, according to EPR quantitative analysis is indicated for the 4,4'-bipyridine case. The spacing among the adjacent layers can be varied and controlled by ion exchange of the chlorine anions with e.g. lauryl sulfate. All 2-D polymers are electrochemically active and stable, as evidenced by the cyclic voltammetry measurements. As such, the π-conjugate organic material displays low band gaps and electrical conductivity in the range of 10-4-10-5 Ω-1cm-1 (300 K). The UV-Visible absorption peaks are red shifted (from 380 to 800 nm depending on the bridging diamine unit) compared to the constituted members (220-250 nm) which indicates an electron delocalization alongside the 2-D network. The AFM and SEM micrographs are revealing the formation of nano or submicron structures. The samples were further characterized with FT-IR spectroscopy, dynamic light scattering and TGA measurements, while the color of the derivatives and consequently the UV-Vis absorption peaks can be varied through simple chemical or electrochemical oxidation.

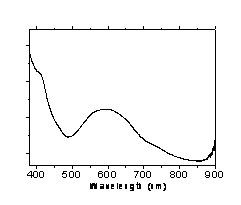
Figure 1. a) SEM image of the 1,4 phenylendiamine derivative b) UV-Vis.spectra of the 4,4 bipyridine derivative
1. Bourlinos A.B., Dallas P., et al. Eur.Polymer.J. 2006, 42, 2940