Main lectures
1 2 3 4 5 6 7 8 9 10
DESIGN OF BLOCK COPOLYMERS FOR NANOSTRUCTURATION
OF EPOXIES AND OTHER THERMOSETS
J.-P. Pascault
Laboratoire des
Matériaux Macromoléculaires/Ingénierie des Matériaux Polymères, UMR
CNRS 5223, Bât. Jules Verne, INSA-Lyon, 69621 Villeurbanne Cedex,
France (Jean-Pierre.Pascault / insa-lyon.fr)
Nanostructured
thermosets, TS may be obtained by the self-assembly of amphiphilic
block copolymers, BCP in a reactive solvent and fixation of the
resulting morphologies by the cross-linking reaction. In particular,
BCP self-assembled into vesicles and micelles can significantly
increase the fracture resistance of cured epoxies with a minimum impact
on Tg and modulus. BCP used for these purposes are composed
of one block that is immiscible in the TS precursors and at least
another one that is initially miscible and does not phase separate
during the network formation. In this way the self-assembled structure
is fixed by the cross-linking reaction. Various immiscible blocks have
been employed to generate stable nanostructures in epoxies cured with
different hardeners. The election of the miscible block is strongly
dependent on the hardener selected to perform the cure. Examples of
miscible blocks are: PEO, PMMA and PCL.
The search of a miscible block for a specific
epoxy-hardener combination is not a trivial task due to the variety of
mechanisms of network formation involving different types of hardeners.
PMMA may be a convenient selection as a miscible block because it is
soluble with epoxy in all proportions. However, for most hardeners it
becomes phase separated during polymerization well before gelation. On
the other hand, poly(N,N-dimethylacrylamide) (PDMA) is miscible both in
non-polar solvents such as cyclohexane and in highly-polar solvents
such as water, methanol and ethanol. Therefore, the family of random
copolymers poly(MMA-co-DMA),
with different proportions of both monomers, should be a useful choice
as a "universal" miscible block for the synthesis of nanostructured
epoxies. Examples of nanostructured epoxies and other TS like
polyurethanes or unsaturated polyesters, and some rules to control
processing and properties will be given in this presentation.
SOL-GEL DERIVED FUNCTIONAL NANOSTRUCTURED INORGANIC AND HYBRID
MATERIALS
C. Sanchez
Laboratoire de
Chimie de la Matière Condensée, Université Pierre et Marie Curie. 4,
Place Jussieu, Tour 54, 5e. 75252, Paris, Cedex 05, France.
* clems / ccr.jussieu.fr
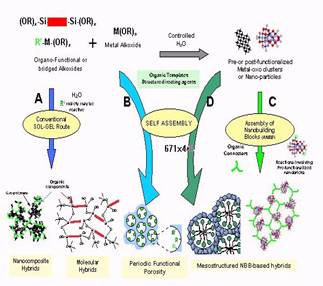
Hybrid
nano-composites materials can be obtained either through hydrolysis and
condensation reactions of functional metal alkoxides or chlorides or
through the assembly of well defined nanobuilding blocks. The
properties that can be expected for such materials of course depend on
the chemical nature of their components but also on the extend and the
nature of their interface. This interface can also be tuned with or
without templates to built nano-structured hybrids or even
nanostructured metallic oxides. The control of the surface properties
of the inorganic nano-building bricks by using nucleophilic groups
carried by texturing agents triggers the obtention of a given
nano-phase. Considerable effort is being currently directed to the
obtention of nanostructured oxides. The use of ordered lyotropic phases
as templating agents (surfactants, organogels, bio-polymers), leading
to a mesoscopically ordered hybrid precursor allow the obtention of
long-range nanostructured hybrid or metal oxide phases shaped as bulks
or films. Some examples concerning the design of hybrid materials made
by using, metal alkoxides precursors or nano-building bricks, to create
mesoscopically ordered phases will be presented together with some of
our results concerning materials having hierarchical structures. Hybrid
Meroporous sensors, photocatalysts and catalysts, new solar and fuell
cells and new mesoporous films made of nanocrystalline multimetallic
metal oxides will be also described.
C.Sanchez and F.
Ribot, New Journal of Chemistry, 18, (1994),1007., G. Soler-Illia, L. Rozes, M.K.
Boggiano, C. Sanchez, C-O. Turrin, A-M. Caminade, J-P. Majoral, Angewandte Chemie, 39,23 (2000), 4249. C. Sanchez and B. Lebeau, MRS Bulletin, (2001), 26 (5), 377. D. Grosso, G.
Soler-Illia, F. Babonneau, C. Sanchez, PA Albouy , A. Brunet, A.R.
Balkenende, Adv. Mater.,
2001, 13,1085..C. Sanchez,
G. Soler-Illia,F. Ribot, T. Lalot, C. Mayer, V. Cabuil, Chem. Mater., October 2001, 13, 10.G. Soler-Illia, C. Sanchez, B.Lebeau, J.
Patarin, Chem. Rev., Nov 2002., D. Grosso, E. Crepaldi,G. Soler-Illia,
B. Charleux and C. Sanchez, Adv. Funct. mater, 2003,13,37.
E. Crepaldi,G.
Soler-Illia, D. Grosso, A. Bouchara and C. Sanchez, Angew. Chemie,
2003, 42,347.
C.Sanchez, G.
Soler-Illia, F. Ribot, D. Grosso Comptes-Rendus Acad Science Chimie,
2003, 8, 109.
E. Crepaldi,G.
Soler-Illia, D. Grosso, F. Ribot, F. Cagnol and C. Sanchez, JACS, 2003.
C. Sanchez et
al ; Nature Materials 2004, 2006, 2005, and Chem mater 2004, Advanced
Functional Materials 2004., *Special Issue on Functional Hybrids : J.
Mater Chemistry, 2005, vol 15, N°35-36.C. Sanchez Guest Editor, ,
* Functionnal
Hybrid Materials, P. Gomez Romero and C. Sanchez, Wiley-VCH, ISBN
3-527-90484-3, 2004
Inorganic-organic hybrid polymers based on surface-modified metal
oxide clusters
U. Schubert
Institute of
Materials Chemistry, Vienna University of Technology, Getreidemarkt 9,
A-1060 Wien, Austria (uschuber /
mail.zserv.tuwien.ac.at,
http://www.imc.tuwien.ac.at)
Carboxylate-substituted
metal oxide/alkoxide clusters of the general formula MxOy(OH/OR)v(OOCR)w
with various compositions, structures, diameters and shapes were
obtained either by reacting metal alkoxides with unsaturated carboxylic
acids or by ligand exchange reactions. In each case, the metal oxide
cluster core - with dimensions between 0.7 and 1.8 nm - is capped by a
variable number of carboxylate ligands, which are fully accessible for
polymerization reactions.
The
carboxylate-substituted metal oxo clusters not only exhibit interesting
coordination chemistry, they are also very versatile nanosized building
blocks for the preparation of inorganic-organic hybrid polymers.
Ring-opening metathesis or free radical polymerization of small
proportions of the clusters with organic co-monomers
(methylmethacrylate, acrylic acid, styrene, norbornene, etc.) resulted
in hybrid polymers in which the clusters crosslink the polymer chains.
Cluster-reinforced
organic polymers constitute a new class of inorganic-organic hybrid
materials with interesting materials properties. Swelling behavior in
organic solvents, thermal stability and mechanical properties of the
cluster-crosslinked polymers are distinctly different to that of the
parent polymers. The materials properties depend on the polymerization
conditions, the cluster proportion in the polymer and - to some extent
- also on the kind of cluster; they originate from a combination of
nanofiller and crosslinking effects.
PERFECT AND NEARLY PERFECT
SILSESQUIOXANE FOR POLYFUNCTIONAL NANOPARTICLES AND NANOCOMPOSITES
R.M. Laine, M. Roll,
M. Asuncion, C. Brick, S. Sulaimann, R. Tamaki
Depts. of Materials Science and Engineering, Chemistry,and
Macromolecular Science and Eng., University of Michigan, Ann Arbor, MI.
48109-2136, U.S.A.
talsdad / umich.edu
Octafunctional
cubic silsesquioxanes [RSiO1.5]8 are unique
molecules wherein the body diagonal of the single crystal silica core
is 0.5 nm and each functional group attached to the vertices of these
cores occupies a different octant in Cartesian space. These materials
are easily accessible in high yields from simple starting materials
including rice hull ash. Furthermore, an extensive variety of
functional groups can be introduced. Consequently, these materials
offer unique opportunities to engineer new nanobuilding blocks and
polyfunctional materials. The nanobuilding blocks provide the tools for
creating novel nanocomposites nanometer by nanometer in 1-, 2- or 3-
dimensions. The polyfunctional materials have unique properties in
their own right.
We describe here methods of making and processing
highly imperfect, slightly imperfect and perfect nanostructures from Q8
[RSiMe2SiO4]8, [RPhSiO1.5]8
(ROPS) and [RPhSiO1.5]12
, (RDPS) systems and some
of their properties including unusual photoluminescence behavior.
a b
b c
c 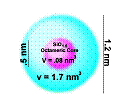
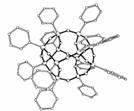
Figure
1. Cubic silsesquioxanes. a. Q8 (Q= SiO4) R= H, vinyl, epoxy,
alcohol, amine, halide, isocyanate, acrylate, etc. b. ROPS,
R = Br, NH2, alkyl, alkene, acetylene, acyl, azo, etc. R =
same or mixed. c. typical
sizes/volumes. d. DPS
For example, both the OPS
and DPS can be halogenated
to give BrxOPS
and Brx,yDPS
and IxOPS and Ix,yDPS (x = 8, y
= 12). The iodo compounds are >90% para
substituted. Using both Heck and Suzuki coupling reactions we have
learned make materials that offer unique photoluminescence behavior
that suggests some semiconducting behavior.
References
1.
R.M. Laine, "Nano-building blocks based on the [OSiO1.5]8
silsesquioxanes," J. Mater. Chem., 15,
3725 - 44 (2005).
2.
C.M. Brick, Y. Ouchi, Y.
Chujo, R.M. Laine, "Robust Polyaromatic Octasilsesquioxanes from
Polybromophenylsilsesquioxanes, BrxOPS, via Suzuki Coupling," Macromol.
38, 4661-5 (2005).
3.
C. Brick, R. Tamaki, S-G. Kim, M.
Asuncion, M. Roll, T. Nemoto, R.M. Laine, Spherical, Polyfunctional
Molecules Using Polybromooctaphenylsilsesquioxanes as Nanoconstruction
Sites," Macromol. Macromol.
38, 4655-60 (2005).
ACHIEVING VALUE WITH NANOSTRUCTURED
CHEMICALS
J.D.
Lichtenhan1*, B. Fu1, P. Wheeler1, R.
Misra2, S. E. Morgan2
1Hybrid Plastics Inc., Hattiesburg, MS USA 39401
www.hybridplastics.com
2Department of Polymer Science, University
of Southern Mississippi, Hattiesburg, MS 39406
The use of
polyhedral oligomeric silsesquioxanes (POSS) for modification of
surface properties of engineering thermoplastics offers low-cost
alternative to fluoropolymers and silicones while maintaining the
processing advantages of an engineering polymer. POSS®
masterbatches in PP, PE, and PA are prepared via twin screw compounding
and can be let-down via single screw extrusion. A 60% reduction in
nanoscale surface friction coefficient relative to base polymer is
observed in PP by incorporating of 10% POSS® into the
polymer. Changes in the surface topography have been characterized by
SEM and nanoprobe atomic force microscopy (AFM) and will be discussed
with respect to the reduction of friction and improvement of
hydrophobicity. Applications for the POSS masterbatches as low friction
textiles, and in sporting goods will be presented.
SMALL-ANGLE NEUTRON SCATTERING AND
DYNAMIC LIGHT SCATTERING STUDIES ON HIGH-PERFORMANCE
POLYMER-NANOCOMPOSITE HYDROGELS
M. Shibayama
Institute
for Solid State Physics, The University of Tokyo, Kashiwanoha, Kashiwa,
Chiba 277-8581, Japan
The structure
of poly(N-isopropylacrylamide) (PNIPA)-clay nanocomposite gels (NC gel)
was investigated in terms of small-angle neutron scattering (SANS). The
NC gels were prepared by radical polymerization of PNIPA in the
presence of clay platelets. The gelation dynamics was also investigated
by time-resolved dynamic light scattering. It allowed us to determine
the percolation threshold and to monitor the critical dynamics.
Contrast-matching as well as contrast-variation methods were employed
to elucidate the local structure of NC gels. It was found that PNIPA
chains are condensed near clay platelets with a thickness of a few nm.
SANS of deformed NC gels revealed that NC gels consist of long PNIPA
chains tied by clay platelets. This unique structure is responsible for
large deformability and high toughness of NC gels.
TAILORING
FUNCTIONALITY OF POLYSILSESQUIOXANES FOR FUEL CELL APPLICATIONS
D.A. Loy
Department of Materials Science & Engineering,
University of Arizona, Tucson, Arizona 85721-0012
Polysilsesquioxanes
are widely used as coupling agents and surface
modifiers due to their hybrid organic-inorganic nature.
There have been a number of groups focusing on developing
electrolyte membranes based on polysilsesquioxanes for fuel cell
applications. In this presentation, I will
provide a brief review of these efforts and describe our own efforts to
prepare highly functionalized materials using tetrasulfide
bridged polysilsesquioxanes for fuel cell membranes and for metal ion
adsorbents. Each tetrasulfide
linkage serves as a template for either two sulfonic acid groups or two
thiol groups.
These groups are automatically positioned adjacent to one another for
proton conduction or metal chelation. For fuel cell membranes we focused on non-porous
membranes in which the tetrasulfide groups
were oxidized to sulfonic acid groups. Ion
conductivity experiments revealed that the resulting membranes did
conduct protons (0.002 S/cm), but that the membranes were more brittle
than desirable. In the metal adsorbent
studies, it was important to retain as much porosity as possible in the
materials. Our best efforts here utilized
surfactant templating to maintain mesoporosity. The
result was high surface area materials with high platinum ion capacity. However, we were surprised to find that the tetrasulfide bridged precursor was almost as
good as the reductively cleaved system at scavenging chalcophilic metals.
NANOSTRUCTURED MATERIALS BASED ON POLYHEDRAL AND
BRIDGED SILSESQUIOXANES
R.J.J. Williams
Institute of Materials
Science and Technology, University of Mar del Plata and National
Research Council, J. B. Justo 4302, 7600 Mar del Plata, Argentina
(williams / fi.mdp.edu.ar)
Monofunctional
polyhedral oligomeric silsesquioxanes (POSS) have been used to modify
different types of polymer networks. In most cases, a
polymerization-induced phase separation takes place leading to a
dispersion of a POSS-rich phase in the polymer network. We will show
that the nature of the organic inert group and the pre-reaction of the
functional group can be used to control morphologies generated in
POSS-modified epoxies. An example where large amounts of a
monofunctional POSS can be introduced to an acrylic formulation without
any evidence of phase separation will be discussed. Mechanical and
thermal properties of the resulting hybrid materials will be analyzed.
Narrow
distributions of POSS can be synthesized in one step starting from
organotrialkoxysilanes bearing hydroxyl groups in beta position with
respect to tertiary amines. When this precursor is co-condensed with
tetraethoxysilane (TEOS), soluble functionalized-silica can be
obtained. This product can be covalently bonded to the surface of
silica and the resulting modified-silica used as a support of a
metallocene catalyst. The activity of the supported catalyst for
ethylene polymerization was similar than the activity found in the
homogeneous reaction.
Bridged
silsesquioxanes can be synthesized by the hydrolysis and condensation
of monomers containing an organic bridging group joining two
trialkoxysilyl groups. When the organic bridge contains urea groups,
the material exhibits photoluminescence arising from processes taking
place both in inorganic and organic domains. We will show that the
photoluminescence spectra can be varied by controlling the relative
rates between the self-assembly of organic bridges and the inorganic
polycondensation. Specific dies can be covalently bonded to the
structure during the synthesis producing a significant variation of the
emission spectra.
When bulky
pendant groups are present in the organic bridge, a nanostructuration
at different levels takes place due to the need to accommodate the
bulky group in the structure. We will discuss examples of the
nanostructures obtained when the pendant group is a dodecyl chain or a
cyclohexyl ring.
ALTERNATING CURRENT ELECTRIC AND
DIELECTRIC PROPERTIES OF NANOCOMPOSITES
M. Matsuo, Y.
Xi, Q. Chen, Y. Bin
Faculty of
Human Life and Environment, Nara Women's University,
Nara,
630-8506 Japan (m-matsuo / cc.nara-wu.ac.jp)
Conductive
polymer composites were obtained by adding conductive fillers like
carbon black (CB), carbon fibers (CFs) or multi-wall carbon nanotubes
(MWNTs) to polymer matrix. The composites by admixing common conductive
fillers were characterized by a percolation threshold or a critical
value at which the electrical conductivity starts to increase as a
function of filler contents. For ultra-high
molecular weight polyethylene (UHMWPE), its high melting viscosity,
limited the use of the melt processing method. The
gelation/crystallization from solution was proven to be more effective
than the other methods to disperse carbon fillers in the UHMWPE matrix.
This talk deals with two contents.
1) UHMWPE and
MWNT composites were prepared using either decalin or paraffin as
solvents. Electrical conductivity measurements were performed for the
original and heat-treated composites. The drastic increase in
conductivity occurred at low MWNT content for the composite prepared in
paraffin, while the conductivity of the composite prepared in decalin
increased slightly up to 10wt% MWNT content. Scanning electron
microscopy observations revealed that the MWNTs within the composite
prepared in decalin were covered by UHMWPE, and their average diameters
were much greater than those of the original MWNTs, while the average
diameter of the MWNTs within the composite prepared in paraffin was
similar to the diameter of the original MWNTs. Such different
morphologies were found to be due to the different crystallizations.
2) Some of
UHMWPE-carbon filler composites showed a sharp increase in electrical
resistivity at elevated temperature close to the polymer melting point,
which is known as positive temperature coefficient (PTC) effect. A
simple resistor-capacitor circuit model was proposed to explore the
alternating current (AC) conductivity and dielectric permittivity
behavior of UHMWPE-CF composites. The composites were considered as a
system composed of random arrays of closely spaced conductors dispersed
in an insulating UHMWPE matrix and broad frequency measurements were
carried out to probe the conducting paths and the space gaps to show
the experimental evidence for explaining PTC.




