RWTH Aachen, Institute for Technical Chemistry and Macromolecular Chemistry, hemocompatible and biocompatible biomaterials, Worringer Weg 1, 52074 Aachen, Germany; baumann (rwth-aachen.de)
More than 40 Years ago about the first development of a thromboresistant surface was reported. Salzmann et al. reviewed in 1994 the work about thromboresistant surfaces as follows: The need of artificial materials that can come in contact with blood without induction of thrombosis has led to exploration of many varieties of materials in the hope of finding some that are thromboresistant. Because of uncertainty with respect to the properties of a surface that determines its reactivity with blood advances in this field have often been empirical or attributed the theoretical consideration of unproven relevance. Ratner reviewed the literature at nearly the same time. His paper was entitled "The blood compatibility catastrophy". "We do not yet have blood compatible synthetic materials. Perhaps 100 or more papers are published each year discussing materials that are labelled 'blood compatible', 'non thrombogenic' or 'antithrombogenic'. It´s my contention that in most cases, they are uncompletely tested." Furthermore there are no focussed and standardized in vitro and in vivo blood tests used and the tests mostly were not done under defined essential flowing conditions (platelet reactivity is strongly dependant of shear rates) in comparison to related natural conditions for developing f.i. microvascular vessels.
In this presentation systematical developments of a well characterized promising athrombogenic coating for microvascular vessels will be reviewed and new results will be added. The coating is based on a polysaccharide (ESHS: endothelial cell surface heparan sulfate, which was isolated from luminal side of blood vessels of living organisms with the highest athrombogenic standard known). The ESHS and additionally synthetic heparinoid mimetics for the domains of the ESHS based on regioselectively modified polysaccharides with heparinlike functional groups (removed from or introduced into Chitosan, Cellulose and Heparin) were also covalently immobilized onto a silicon tube of 3 mm diameter by a monolayer. The coating was extensively analysed by surface spectroscopic and chemical methods. Different in vitro blood tests under flow conditions were developed and one with the best correlation to the in vivo blood test (AV-Shunt, sheep) without using anticoagulants was standardized for biomaterials The ESHS showed no relevant complement activation and no toxicity, it can be steam-sterilized without changing the molecular weight and the functional groups. A few heparinoids are also promising candidates for athrombogenic coatings. Depending on regioselectivity the coatings are able to suppress adhesion of selected plasma proteins, which would normally activate the receptors of platelets on surfaces. These studies give also a primarily insight in the mechanisms of athrombogenicity and the interaction of blood with regioselectively modified polysaccharidses on biomaterial surfaces as well in nature.
Literature:
Gott VL, Koepke DF, Dageff RL et al., The coating of intravascular plastic prothesis with colloidal graphite, Surgery 1961; 50: 382.
RatnerBD, The blood compatibility catastrophe, J.Biomed. Mat. Res. 1993; 27: 283.
Salzmann EW, Merill EW, Kent KC, Interaction of Blood with artificial Surfaces in Hemostasis and Thrombosis. Basic Principles and Clinical Practice, Eds.: Salzmann RW, Hirsch J, Marder VJ and Salzmann E, J.B. Lippincott Company, Philadelphia 1994: 1469.
Baumann H, The role of regioselectively sulfated and acetylated polysaccharide coatings of biomaterials for reducing platelet and plasmaprotein adhesion, Sem. Thromb. Hem. 2001; 27(5): 445.
Baumann H, Keller R, Baumann U, Development of Microvascular Artificial Platelet Inert Blood Vessels, Basic Principle and Animal Experiments, Frontiers in Biomedical Polymer Application, ed.: Ottenbrite, Technomic Publishing Lancaster, Basel 1999: 159.
EcoleNationale Superieure de Chimie et Physique de Bordeaux (ENSCPB-LCPO). 16, Pey Berland. 33607 Pessac, France.
The design and preparation of complex nanostructured materials has been motivated to a large extent by the need to control the composition, structure, and function of the molecular entities. Control of the macromolecular nanoscale size has been achieved by two main strategies. First, via covalent incorporation of branches obtaining dendrimers, dendrigraft or arborescent graft polymers. This strategy suppose normally large and tedious synthetic procedures. The second more straightforward alternative concerns the use of the non-covalent interactions to induce self-assembly of a large number of small molecules.
Our group has focused in the preparation of diblock copolymers where one or both blocks are peptides and the analysis of their properties mainly in solution. From these points of view we were able to prepare hybrid PB or PI and polypeptide (L-glutamic acid or L-lysine) block copolymers that form spherical micelles, vesicles and inverse micelles depending on the relative composition.

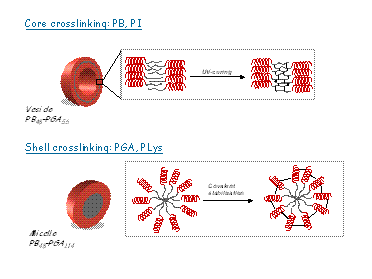
Otherwise, one of the most important drawbacks of these supramolecular assemblies objects is its poor stability. In order to enhance the stability of the nano-aggregates these can be covalently captured, for example, via crosslinking if one of the blocks contains crosslinkable groups. We have succeed in the preparation of stable nano-objects where the core or the shell have been crosslinked. The resulting nano-objects associated with peptide stimuli-responsive effects may be of potential interest for a variety of applications including transport, storage/release or catalysis.
FIG1.- Nano-objects stabilization via core and shell crosslinking strategies.
PRESTO, JST, and Graduate School of Engineering, Osaka University, 2-1, Yamada-oka, Suita 565-0871, Japan
In order to elucidate and mimic the versatile functions of DNA and RNA, a wide variety of nucleic acid model have been designed. An inherent, crucial drawback of these model systems is the lack of a direct means to actively control the function of these nucleic acids.
It is well documented that the recognition/binding abilities of nucleic acids and their analogues are critically affected by their conformational variations, especially through the syn-anti orientational change of nucleic acid base moiety. Certainly, the anti orientation of nucleobase is an essential factor for efficient recognition, since syn-orientated nucleobases are unfavorable for forming intermolecular hydrogen bonds with the complementary base. Thus, if nucleobase orientation could be switched by an additive, external control of recognition behavior can be readily applied. Unfortunately, no external agent or factor, which affects the syn-anti orientation and therefore the recognition behavior of nucleic acids and analogues, has been found or proposed to date, although the unusual syn orientation is known to be induced by several internal factors, such. Indeed, ketalization of the cis-2',3'-diol of uridine enhances the syn/anti ratio through the imposed 2',3'-planar-O4'-exo-furanose structure. However, the energetically disfavored syn orientation has not been induced by external agents.
It is also well known that boric acids form cyclic esters with a variety of cis-1,2-diols, including sugars and ribonucleosides, and this esterification process is reversible in aqueous solutions at moderate pH. Recently, we have reported that the nucleobase orientation of 5'-aminouridine is switched from anti to syn simply by using borates as an external agents, as determined by circular dichroism (CD) and NMR-NOE spectroscopy. This unprecedented orientation switching phenomenon is expected to provide us with a unique methodology for externally controlling nucleic acid recognition, if a nucleic acid analogue, in which these functionalities are carrying a ribose unit with free 2',3'-diol and 5ユ-amino/amide proton, can be prepared, since both is indispensable to the orientational switching process. For this purpose, we wish to propose a new category of nucleic acid analogues, i.e. peptide ribonucleic acid (PRNA), in which the ribonucleoside units are not directly incorporated into the main chain, as is the case with RNA, but are instead attached to the peptide backbone as pendant groups. In the present study, 5'-aminouridine and 5'-aminocytidine units are tethered to the isopoly(L-glutamic acid) backbone at the remaining a-carboxyl group of glutamic acid through the 5'-amino group, thus reserving the ribose cis-2',3'-diol for cyclic borate formation as well as the 5'-amide proton for hydrogen bonding with the pyrimidine nucleobase 2-carbonyl. The results obtained in the study are encouraging, demonstrating that a- and g-PRNA form stable hybrid complexes with the complementary oligonucleotides, which are readily dissociated upon addition of borax. Certainly, a definition of the scope and limitations of this strategy remain to be addressed, including the study of sequence selectivity and the search for other switching devices/agents.
Ref.; H. Sato, T. Wada, Y. Inoue, Tetrahedron, 59, 7871(2004); T. Wada, N. Minamimoto, Y. Inaki, and Y. Inoue, J. Am. Chem. Soc., 122, 6900 (2000); T. Wada, H. Sato, and Y. Inoue, Nucleic Acids Res. Suppl., 29, 299 (2001); T. Wada, N. Minamimoto, Y. Inaki, and Y. Inoue, ChemLett., 1025 (1998).
aTechnical University of Szczecin, Polymer Institute, P-70322 Szczecin, Poland, e-mail: mirfray (ps.pl)
bUniversity of Bayreuth, Friedrich Baur Research Institute for Biomaterials, D-95440 Bayreuth, Germany, e-mail: martina.feldmann (fbi-biomaterialien.de)
Synthetic polymers are especially attractive for the preparation of various composite materials, and in combination with bioactive components, such as bioglasses, hydroxyapatite or tricalcium phosphate TCP, they represent a novel class of bioactive hybrid materials. Recently, elastomeric copolymers of poly(butylene terephthalate) (PBT) and dimer fatty acid (DFA) have been evaluated as candidate materials for the preparation of temporary tendon prosthesis. These polymers (PED) have sufficient mechanical properties and excellent flexibility. To enhance their bioactivity and expected bone-bonding properties, various polymer/TCP composites can be prepared.
Preparation method for hybrid organic/inorganic materials and evaluation of their in vitro properties were investigated. It could be shown that the in vitro cytocompatibility is improved by adding TCP up to 20 vol% highly significant(Fig. 1).
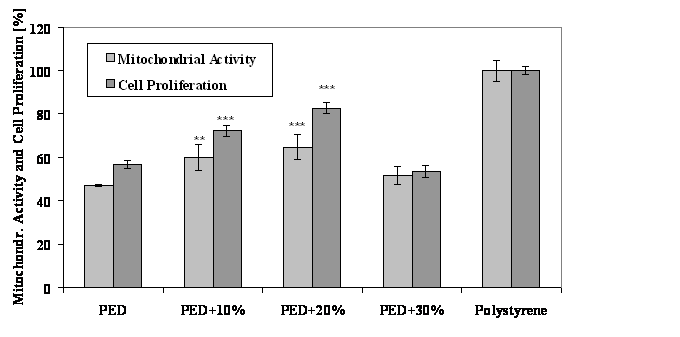
Fig. 1: Mitochondrial activity and proliferation of L929 cells after 24 hours on PED-polymer/TCP composites compared to the unmodified polymer and to polystyrene
Department of Materials Science and Engineering, University of Naples Federico II, P. le Tecchio 80, Naples, 80125, Italy, branda (unina.it)
As it is known, hydrogels are polymeric networks held together by cross-links of either weak cohesive forces such as hydrogen or ionic bonds. They imbibe large quantities of water or organic liquids by swelling without dissolving. It is known that the swelling property is very important for many applications. Polyelectrolytes are hydrogels that contain relatively high concentrations of ionisable groups along the backbone chain. Recently new anionic and new cationic polyelectrolyte p(HEMA) hydrogels were synthesized by copolymerisation of 2-hydroxyethyl methacrylate (HEMA) with the anionic monomer 2-acrylamido-2-methylpropane-sulphonic acid (AMPS) and 2-methacryloyloxyethyltrimetil ammonium chloride respectively.
Samples of p(HEMA) and of the above indicated polyelectrolytes, cross-linked with ethylene glycol dimethacrylate (EGDMA), were submitted to the biomimetic method in order to obtain an Hydroxyapatite (HA) coating. Following this method the support is dipped together with a glass of suitable composition into a simulated body fluid (SBF), of composition close to the blood plasma, in order to promote HA nucleation on the substrate; the following dipping into 1.5 SBF, having ionic concentrations increased by 50%, has HA crystals grown to a dimension that can be controlled by properly selecting the temperature and time of the treatment.
The method was successfully used to obtain HA micro and nanostructures on the above indicated hydrogels. The HA coating obtained by means of the biomimetic method was checked by means of SEM, EDS microprobe elemental analysis, FTIR infrared spectroscopy and X-ray photoelectron spectroscopy (XPS). It was proved that HA formed also on the surface of the internal pores. The coating had a very good adhesion to p(HEMA). Moreover, good adhesion of osteoblasts on this coating was measured. The method changed also the swelling behaviour. The mechanism of formation of HA coating was investigated.
Department of Fibre and Polymer Technology, Royal Institute of Technology, Teknikringen 58, SE-100 44 Stockholm, Sweden, E-mail: aila (polymer.kth.se)
Artificial tissue implants are developed from bioresorbable polymeric scaffolds. The structure and function of the engineered tissue is controlled in a pre-designed manner.
Copolymerization of monomers is used to combine favorable properties of different monomers. Hydrophilicity, mechanical properties, and even degradation characteristics are tailored by selecting the proper monomer. The monomers are copolymerized into random copolymers and into different block copolymers, such as diblock-, triblock-, and multiblock copolymers, both linear and branched.
Grafting techniques are used for the synthetic modification of polymer surfaces, typically involving two separate steps. Firstly, the polymer groups exposed on the surface are activated via plasma or electron beam-irradiation. In a second step, the activated groups are allowed to react so that new oligomeric or polymeric side groups, i.e. grafts, are covalently attached to the polymer surface.
Biodegradable polymers with nano or submicron dimensional surface patterns are made with various techniques such as:
Results from blockpolymers such as poly(L-lactide-b-1,5-dioxepan-2-one-b-L-lactide) (PLLA-PDXO-PLLA) and random copolymers of e-caprolactone (CL) and 1,5-dioxepan-2-one (DXO) will be presented.
Laval University, Dept of Materials Engineering, Bioengineering and Biomaterials Laboratory & Research Center, St-François d'Assise Hospital Pavillon Pouliot, Quebec City, Canada
The specific scaffolding of cells for vascular tissue and organ regeneration is a major concern in tissue engineering. Thus, the scaffold have to guarantee both, a well-suited substrate to cell activity, and a mechanical-optimized support leading to tissue and eventually organ regeneration. To develop a structure which show mechanical properties close to those of the arteries is not a trivial matter. In fact, it is well know that synthetic arterial substitutes, such as Teflon and Dacron prostheses, are much more rigid then natural arteries, their longitudinal and radial (visco-)elastic modulus being 10 times higher. Therefore, it is evident that a mismatch does occur between the ePTFE prosthesis elasticity and that of the biological vessel when the prosthesis is implanted. However, tissue-engineered vessels strength and the ability to withstand physiological arterial pulsing pressures are clearly insufficient and they fail when implanted in animals for long periods. Longer culture periods, more uniform cell colonization, and treatment of culture media with cytokines to stimulate collagen production are envisaged to improve the mechanical properties of the tissue-engineered prosthesis; however, to date, no relevant results appear to have been achieved. Therefore, the aim of this work was to develop a scaffolding structure, with selective porosity, to provide the ideal substrate on which cells will be encouraged to migrate by mechano-transduction.
In this presentation, we will focus on the development of scaffolds made of porous polypropylene cylindrical structure or cross-linked collagen closely matching the mechanical properties of arteries. The complete chemical and mechanical characterization of these scaffolds was carried out, as well as fibroblasts and endothelial cell culture assays will be presented.
aDepartmentof Materials Science, Saarland University, 66123 Saarbrücken, Germany
bDepartmentof Biopharmaceutics and Pharmaceutical Technology, Saarland University, 66123 Saarbrücken, Germany
cDepartmentof Pharmaceutics, Philipps University Marburg, 35032 Marburg, Germany
Cell-surface interactions are fundamental aspects to understand the role of surface chemistry and topography of biomaterials. If cells differ in their response to topographic variations, this phenomenon may be exploited to regulate cell functions and design implant materials. Therefore, controlling the growth and spatial orientation of cells has become an important investigation activity of biomaterials.
This presentation will introduce laser interference micropatterning (LIP) to prepare periodic linear, protuberant and holey micropatterns ranging from submicrometer to 20 mm for study of cell-surface interactions. This new process provides a straightforward micropatterning technique based on selective laser ablation of polymers utilizing the periodic energy distribution of two or more beam interferencepatterns. AFM micrographs show that the micropatterns on poly(ethylene terephthalate) (PET), polycarbonate (PC) and Thermanox® are well-defined and of great consistency in a large area. The surface topographical, chemical and physical properties associated with cell adhesion were systematically characterized. Together with analysis of the small molecules and deposited polymer segments produced during laser ablation, the ablation mechanism is discussed. Because of the versatility of lasers, there is in principle no limit in the choice of polymers.
Human pulmonary cells cultured on laser- micropatterned PET, PC and Thermanox surfaces are elongated, spindle-like shaped. In addition, the cells show directional growth parallel to the linear patterns with all different groove widths. In contrast, cells cultured on unmodified film are larger, multipolar, and rather randomly orientated, and frequently overlap each other. Further investigations found that human pulmonary fibroblast cells cultured on linear and protuberant micropatterns induced by LIP show inflammatory response when the spacing falls within definite range, which has not been reported by cell adhesion on any other micropatterns. In contrast, cells seeded on micropatterns with larger spacing and unmodified films do not show any green fluorescent signal. Since all the films were coated with collagen, the E-selectin expression should be trigged only by surface micropatterns and topography in smaller region regardless to the possibly chemical modification of the micropatterned surface.
aDepartment of Pathology, Vanderbilt University School of Medicine, Nashville, TN 37232-2562,
bChemical Engineering Department, Vanderbilt University, Nashville, TN 37235, USA,
cResearch Service, Department of Veterans Affairs Medical Center, Nashville, TN 37212-2637 and
dDepartment of Microbiology and Immunology, Vanderbilt University School of Medicine, Nashville, TN 37232-2363
E-mail of the corresponding author: ales.prokop (mcmail.vanderbilt.edu)
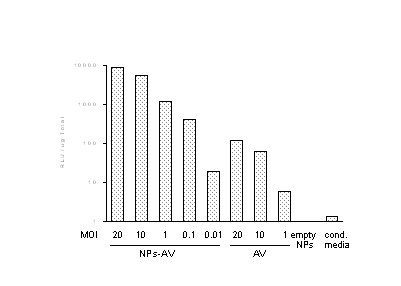
We have developed a series of biocompatible, nanoparticulate formulations that have been designed to retain, protect, and deliver adenoviral gene constructs over an extended time course. These devices can be administered locally or systemically with low toxicity. We have validated the efficacy of nanoparticulate delivery in cell systems which are normally refractory to gene transfer. These targets include pancreatic islets and antigen-presenting cells. We explain our data on the basis of a secondary, receptor-mediated uptake system that permits adenoviral particle release within the transfected cells. Supported by the NIH/NIBIB and the DVA.
An example of gene transfer into dendritic cells is below: luciferase expression in relative units, 3 days post-infection, as compared to free AdV. NPs‑AV - nanoparticles loaded with adenovirus (AV), AV - free adenovirus, empty NPs - no adenovirus, cond.media - only conditioned medium used.
BIORESORBABLE TEXTILE MATERIALS FROM BIODEGRADABLE POLYESTER BLENDS CONTAINING SYNTHETIC ANALOGS OF PHB
M. KOWALCZUKa, M. BEROa, P. DOBRZYŃSKIa, G. ADAMUSa, W. SIKORSKAa, A. ENEb, M. SCANDOLAc
aCentre of Polymer Chemistry, Polish Academy of Sciences, M. Curie-Skłodowskiej 34, 41-819 Zabrze, Poland; cchpmk (bachus.ck.gliwice.pl)
bResearch - Development National Institute for Textile and Leather, Lucretiu Patrascanu 16, 74674 Bucharest, Romania
cChemistry Department 'G. Ciamician', University of Bologna, Via Selmi 2, 40126 Bologna, Italy
It is known that surgical interventions on internal organs are accompanied by problems of wound closure. A very promising method for eliminating or reducing post-surgical tissue adhesion seems to be use of bioresorbable polymer materials. Biodegradable and biocompatible polyesters have attracted much attention by polymer and material scientists owing to their potential capability to be applied as wound dressing, surgical implants and other medical devices. Depending on the application foreseen, the physical properties of such biomaterials may be tuned to match the specific application requirements.
The novel surgical textile materials (BIOMIXED®), derived from blends ofbiodegradable and biocompatible polyesters including synthetic analogues of biopolymers, will be presented (Fig. 1).

Fig. 1
The materials are consisted of atactic poly[(R,S)-3-hydroxybutyrate] as well as poly(L-lactide) and/or its copolymers. The results of evaluation of the structure of these polymer materials at the molecular level will be discussed.