Center for Biomedical Engineering NE47-379 and Center for Bits & Atoms, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139-4307, USA. e-mail: Shuguang (mit.edu), http://web.mit.edu/lms/www/
Two complementary strategies can be employed in the fabrication of molecular biomaterials. In the 'top-down' approach, biomaterials are generated by stripping down a complex entity into its component parts. This contrasts with the 'bottom-up' approach, in which materials are assembled molecule by molecule and in some cases even atom by atom to produce novel supramolecular architectures. The latter approach is likely to become an integral part of nanomaterials manufacture and requires a deep understanding of individual molecular building blocks, their structures, assembling properties and dynamic behaviors. Two key elements in molecular fabrication are chemical complementarity and structural compatibility, both of which confer the weak and noncovalent interactions that bind building blocks together during self-assembly. Significant advances have been achieved at the interface of nanomaterials and biology, including the fabrication of nanofiber materials for three-dimensional cell cultures and tissue engineering, the peptide nanotubes for stabilizing membrane proteins and nanocoating molecular and cell organizations. Molecular fabrications of nanobiomateirals have fostered diverse scientific discoveries and technological innovations.
Departments of Materials and Chemistry, University of California, Santa Barbara, California, 93106, USA, tdeming (mrl.ucsb.edu)
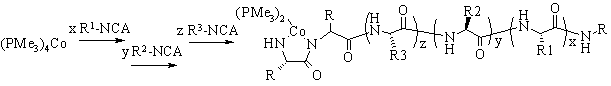
The use of low-valent metal complexes for the polymerization of alpha-amino acid-N-carboxyanhydrides (NCAs) will be presented (Figure 1). Using these initiators, we have prepared block copolypeptides containing a variety of both hydrophilic and hydrophobic domains. The hydrophilic chains are composed of either cationic, anionic, or custom non-ionic residues and the hydrophobic chains are composed of natural non-polar amino acid residues such as leucine, valine and phenylalanine. We have focused our efforts on the self-assembly of block copolypeptides in solution, primarily employing water as the solvent. By working with polypeptides, we expected that the secondary structures present in the block domains would substantially alter the structures of the polymers. The controlled aggregation of block copolypeptides into discrete ordered structures would yield materials valuable for biomedical and materials applications. Examples would be drug and gene delivery, where the shape of the complexes would favor selective interactions with different biological surfaces. Two distinct classes of amphiphilic block copolypeptides, vesicle and hydrogel formers, will be discussed.
 |
| Figure 1: Block copolypeptide synthesis using cobalt initiator |
National University of Singapore Engineering Drive 1, Singapore 119260
Tissue formation within the body, as part of a development, healing and/or repair process, is a complex event in which cell populations in combination with extra cellular matrix self-assemble into functional units and ultimately into tissues and organs. There is intense academic and commercial interest in finding methods to stimulate and control these events and eventually to replicate these events outside the body as close as possible. This interest has accelerated and resulted in that tissue engineering (TE) has become a well recognized research area in the arena of regenerative medicine. TE strategies aims at the regeneration of tissues and restoration of function of tissues/organs through implantation of scaffold/cell or scaffold/neotissue constructs or stimulating cells to grow into implanted tissue conductive and/or inductive matrix systems. The aim of this talk is to review the issues of importance to scaffold design and fabrication. In addition, the current technological and academic challenges and restrictions are broad up and discussed.
The first generation of cell based tissue engineering concepts in the area of skin, cartilage, bone and bone marrow regeneration were derived by isolation, expansion and implantation of cells from the patients own tissue. Even so these concepts can claim a clinical success in selective treatment concepts, yet tissue engineers need to overcome major challenges to allow a widespread along with a clinically safe and predictable application. One challenge is to present the cells in a matrix to the implantation site to allow the cells to survive the wound healing contraction forces, tissue remodeling and maturation. Hence, nowadays a great number of tissue engineering strategies are based of the development of a scaffold/cell construct.
Department of Bioengineering, Rice University, 6100 Main St., Houston, Texas, 77251, USA
The loss of orthopaedic tissues to disease, trauma, or congenital defects often requires surgical intervention to restore function. Current clinical treatments, however, possess significant disadvantages such as limited tissue availability and significant donor site morbidity. Tissue engineering seeks to treat tissue loss by the implantation of a biodegradable material as a carrier for cells and/or bioactive factors to encourage regeneration of the afflicted area. Ideally, such a material would cure in situ to allow for minimally-invasive implantation of the construct.
To this end, our laboratory has recently developed synthetic, in-situ crosslinkable hydrogel materials based on oligo(poly(ethylene glycol) fumarate) (OPF) as injectable carriers for orthopaedic tissue engineering. Using a thermal radical initiator pair, these materials form gel-like specimens within 10 minutes at 37 °C.
The work described in this presentation focuses upon the investigation of the swelling and mechanical properties, as well as the cytocompatibility of this novel polymer system. Further topics include characterization of in vitro release kinetics for various agents, including plasmid DNA and growth factors, from hydrogels demonstrating varied swelling properties. Additionally, the response of stem (marrow stromal) cells embedded within OPF hydrogels, with particular attention to bone formation from these encapsulated cells, is discussed.
Overall, this work demonstrates the great potential of OPF hydrogels as injectable carriers for delivery of cells and bioactive factors to a variety of complex orthopaedic defects.
University of Pennsylvania, 112 Towne Bldg., Philadelphia, Pennsylvania, 19104, USA
Viruses protect, target, and deliver active agents to cells and generally have quasi-spherical and filamentous morphologies. Diblock copolymer amphiphiles can assemble in water into similar shapes, namely vesicles (or polymersomes) and worm-like micelles, that prove especially robust.
Controlled release polymersomes were prepared with the hydrolysable block copolymers poly(ethyleneglycol)-poly(lactic acid) (PEG-PLA) and PEG-poly(caprolactone) (PEG-PCL). When blended with non-degradable diblocks, release reflects a highly quantized process in which any given vesicle is either intact, retaining its encapsulant, or the vesicle is porated and slowly dissolving. Poration occurs as hydrophobic PLA chains are scissioned hydrolytically, progressively generating an increasing number of micelle-forming copolymers in situ. These partially degraded chains eventually congregate above a critical concentration, C*, and locally achieve their desired spontaneous curvature by forming a pore much like added detergent does. PEG-PLA thus sensitizes tough membranes for controlled release. In vivo studies demonstrate the stealthiness of polymersomes, while emerging tests of these vesicles in cell culture demonstrate great promise for controlled release of drugs and oligonucleotides into various cell types.
To understand the dynamics and other properties of the robust, polymeric worm micelles, fluorescence labeling and imaging are being used. Similar diblock copolymers are being studied as with the vesicles, although the worm micelle formers have more symmetric proportions initially. The goal is to exploit these micelles for fluid transport and delivery of the many hydrophobic drugs to cells. In vitro studies demonstrate the degradation kinetics as well as the great flexibility and targetability of these self-assembled micelles. Surprisingly, initial in vivo studies show that microns-long worm micelles circulate in the blood stream for days longer than even the longest circulating 100 nm PEGylated vesicles.
Collectively, the studies demonstrate that getting the amphiphilic proportions correct for diblock copolymers leads to the same richness in aggregate morphology as seen for small surfactants. However, the degradable robustness introduced by the PEG-polyester copolymers makes these tenable mimics of natural viruses.
Department of Innovative and Engineered Materials, Tokyo Institute of Technology 4259 Nagatsuta, Midori-ku, Yokohama 226-8502, Japan, and Polymer Chemistry Laboratory, RIKEN Institute, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan
This paper reports our recent progress on a microbial system for efficient production of biodegradable polyesters of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate], P(3HB-co-3HHx), and ultra-high-molecular-weight P(3HB) using genetically engineered bacteria. We have developed a fermentation technique to achieve high cell density cultivation of recombinant bacteria Ralstonia eutropha and Escherichia coli using inexpensive soybean oil or glucose as a sole carbon source. The use of polyhydroxyalkanoate (PHA) synthase (PhaC) mutants having some amino acid substitutions was able to vary the copolymer composition of P(3HB-co-3HHx) synthesized from soybean oil by the recombinant bacteria . On the other hand, it is also important to understand the enzymatic degradation process of PHA materials for establishing a method to regulate the rate of biodegradation. The enzymatic degradation process of P(3HB-co-3HHx) thin film using an extracellular PHB depolymerase was studied by in situ atomic force microscopy (AMF) in buffer solution, which have suggested that PHA depolymerase predominantly degrades the less-ordered molecular chain-packing regions along the crystallographic a-axis.
Biodegradable P(3HB) fibers with high tensile strength were processed from ultra-high-molecular-weight P(3HB) by a combination with cold-drawing and two-step-drawing, and the distribution of molecular structures in mono-filament was analyzed by micro-beam X-ray diffraction in synchrotron radiation. Strong P(3HB) fibers, with tensile strength of 1.34 GPa, elongation to break of 35%, and Young's modulus of 18.1 GPa, were processed by the cold-drawing from amorphous preform at the near to glass transition temperature and the two-step-drawing at room temperature. The molecular and higher-ordered structures in the P(3HB) mono-filament were analyzed by a micro-beam X-ray diffraction.