1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
DEGRADATION AND STABILISATION OF STYRENE-ETHYLENE-BUTADIENE-STYRENE (SEBS) BLOCK COPOLYMER
N.S. ALLENa, M. EDGEa, A.N. WILKINSONa, C.M. LIAUWa, D. MORELATOUa
E. FONTANb, V. RUIZb, J. BARRIOb, M.A. MARTÍNEZ-ZAPORTAb
aChemistry and Materials
Faculty of Science and Engineering, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, U.K.
bRepsol S. A., 183 Embajadores, 28045 Madrid, Spain
Hydrogenated poly[Styrene-b-butadiene-b-styrene] or poly[Styrene-b-(ethylene-co-butylene)-b-styrene], (SEBS) is one of the more recent block copolymer materials that have found widespread applications as thermoplastic elastomers. The unstabilised polymer is known to undergo degradation during thermal processing and long-term ageing. Coupled with these processes is a marked increase in thermal discolouration. In this work, a detailed spectroscopic analysis has been undertaken on experimental grade SEBS materials using UV, luminescence and FTIR spectroscopy coupled with crosslinking and hydroperoxide analysis in order to understand the nature of these processes. High temperature oxidation results in chain scission and severe crosslinking giving rise to extensive discolouration. FTIR analysis shows complex degradation processes with distinct features associated with each phase. There is a solvent soluble clear phase showing oxidation due primarily to the aliphatic part with a predominant absorption associated with terminal carboxylic acid groups at 1713 cm-1. There is also a solvent insoluble phase, which is predominantly crosslinked aliphatic material with a small inter-phase absorption associated with the aromatic mono-substitution C-H absorption at 1735 cm-1. Thus, end group oxidation is a predominant process with the luminescence showing a rapid initial disruption of the polystyrene excimers coupled with the formation of long wavelength emitting polyconjugated chromophores, possibly some stilbene type in nature. Colour is evident is both crosslinked and uncrosslinked phases. The former shows evidence for the presence of unsaturated carbonyl products and vinyl absorption. Phosphorescence analysis also indicated the presence of initial acetophenone chromophores, which are associated with polystyrene end groups formed by chain breakage at the aliphatic links. These species can act as initial active sensitive sites for further breakdown, possibly via a thermally induced hydrogen atom abstraction process showing a rapid disappearance during ageing. The presence of phenolic antioxidants and phosphites appear to exhibit a powerful effect in synergistically inhibiting the discolouration and oxidation processes. Mechanisms are proposed and discussed for each phase oxidation.
DEGRADATION OF STYRENE-BUTADIENE (SBS) BLOCK COPOLYMER WITH HIGH STYRENE CONTENT
A. BARCELONAa, N.S. ALLENa, M. EDGEa, A.N. WILKINSONa
V. RUIZb, C. GALANb, I. CAMPOb
aChemistry and Materials, Faculty of Science and Engineering, The Manchester Metropolitan University, Chester Street, Manchester M1 5GD, U.K.
bRepsol YPF, Carretera Extremadura, N-V. Km 18, 28931 Mostoles, Madrid, España
The polystyrene-block-polybutadiene-block-polystyrene (SBS) copolymer is a thermoplastic elastomer, widely used in the plastics, coatings and polymer composite industries. The thermal degradation and photo degradation of the unstabilised polymer has been studied using a variety of analytical and spectroscopy methods, including FT-IR spectroscopy, luminescence spectroscopy and colour measurements coupled with sol/gel analysis in order to understand the nature of the processes involved. Changes in the FT-IR spectra show strong absorption associated with carboxylic and/or aliphatic esters at 1717 cm-1 for samples thermally degraded and 1716cm-1 for samples that were photo degraded. Other species such as anhydrides, lactones, peracids and α,β-unsaturated carbonyl species are also formed. The fluorescence shows a rapid disruption of the polystyrene excimers. The increase in colour shows evidence of the presence of unsaturated carbonyl products. The high temperature oxidation results in crosslinking reactions, while in photo oxidation chain scission reactions are predominant, except for samples photo degraded at 254nm where chain scission reactions and crosslinking reactions occur simultaneously.
PHOTODEGRADATION OF COLLAGEN MATERIALS
A. SIONKOWSKA
Faculty of Chemistry, Nicolaus Copernicus University, Gagarin 7, 87-100 Toruń, Poland
*E-mail: as chem.uni.torun.pl
Everyone is exposed to sunlight to a greater or lesser extent and photoageing due to repeated exposure to the sunlight’s UV radiation causes premature skin ageing or photoageing, which is manifest as wrinkles, modified pigmentation, abnormal elastin bundles and a loss of collagen, the major protein of skin. The collagen materials are also exposed to sunlight or even to UV light during sterilization process.
The aim of this work is to study the basic mechanisms by which UV affects collagen at the molecular level to provide an understanding of photodegradation of collagen materials.
Collagen type I from rat tail tendon (RAT collagen) was irradiated with different dose of UV light. The following methods have been employed to assess the changes after UV irradiation: spectrophotometry (UV-VIS, FTIR, spectrofluorimetry, ESR), differential scanning calorimetry, electron microscopy, SDS-PAGE, amino acid analysis, viscosimetry, atomic force microscopy AFM).
The results show that:
Acknowledgement
This work was supported by the Committee of Scientific Research, Poland, grant 3 P05A 06922.
PHOTOCHEMICAL STABILITY OF COLLAGEN/POLY (ETHYLENE GLYCOL) BLENDS
A. SIONKOWSKA*, H. KACZMAREK, J. SKOPIŃSKA, M. WISNIEWSKI,
Faculty of Chemistry, Nicolaus Copernicus University, Gagarin 7, 87-100 Toruń, Poland
*E-mail: as chem.uni.torun.pl
Studies of the phase-structure relationship of polymer blends are attracting a great deal of interest, specially in view of their possible use as new material. The most important is qualitative and quantitative analysis of polymer mixtures in order to evaluate the compatibility of blends of two polymers. Recently the mixtures of collagen with synthetic polymers, and also with other natural polymers are more and more interesting for scientists and technologists [1-2]. Collagen is the most abundant biopolymer in animals where it provides the principal structural and mechanical support [3]. Collagen provides an excellent basis for biomaterials such as arterial prostheses and artificial skin [4].
The aim of this work was to study the photochemical stability of binary blends made of collagen and poly(ethylene glycol ) PEG.
Blends of collagen and PEG have been prepared mainly in the form of films using the solution casting method. The physico-chemical and photochemical stability of the films were investigated by FTIR, UV-VIS spectrophotometry, thermal analysis and viscosimetry measurements.
Our results indicate that collagen and PEG are immiscible. However, collagen/PEG blends have specific interaction between the polymers, modifying their photochemical stability. The mechanisms of reactions occurring in collagen/PEG blend during UV-irradiation have been discussed.
This work was supported by the Committee of Scientific Research, Poland, grant 3 P05A 06922.
References
degradation of water-soluble polymers: photoageing of poly(ethylene oxide)
S. MORLAT , J.L. GARDETTE
Laboratoire de Photochimie Moléculaire et Macromoléculaire, UMR CNRS 6505, Université Blaise Pascal, 63177 Aubière cedex France
e-mail : sandrine.morlat univ-bpclermont.fr
Water-soluble polymers are potential contributors to environmental problems. After use, depending on their characteristics and particular applications, they are discarded as dilute aqueous solutions or into solid waste disposal systems. In the aim to better understand the evolution of the aquatic environment, the mechanism of degradation in solid state was previously determined. Results obtained for irradiated aqueous solutions were then compared.
![]()
Poly(ethylene oxide) (PEO) was irradiated under long wavelengths (l >300 nm) in presence of oxygen either at 20°C in aqueous solution or at 35°C in solid state. Photoproducts have been identified by FTIR analysis coupled to chemical treatments. Irradiated aqueous solutions were characterised by viscometry and size exclusion chromatography (SEC), which showed that photooxidation was leading to a dramatic decrease of the molecular weights. The influence of the pH of the aqueous solution was also examined. Unexpectd results were obtained for the pH 12 solution, indicating a strong inhibition of the oxidation.
Comparison of the results obtained in both states revealed that no direct transposition of the knowledge concerning the behavior of the solid polymer could be made.
[1] S. Morlat and J.L.Gardette, Polymer 42(14) (2001) 6071-6079.
SYNTHESIS AND CHARACTERISATION OF NANOSTRUCTURED STARCH FORMIATES
T. DIVERS*, Y. GROHENS, J.F. FELLER
Laboratoire Polymères et Procédés, Centre de Recherche, rue de Saint-Maudé, BP 92116 Lorient cédex, France
Many researches have been conducted on starches in the field of materials. Indeed, it could replace synthetic polymers, which are currently used. Thus, it could permit, on the one hand to obtain biodegradable materials and on the other hand to keep our petroleum resources.
Nevertheless, the hydrophilic nature of starch is the major drawback to the development of starch-based materials. Therefore, native starch has to be modified in order to increase its hydrophobicity.
The aim of our research is to synthesize a chemically-modified starch with controlled nanostructure by reaction with formic acid. This will allow to achieve the reached biodegradable material by extrusion of this ester of starch with another biodegradable synthetic polymer.
First of all, starch formiates were synthesized by reaction between wheat starch and formic acid diluted in water, leading to starch formiates with low degree of substitution (DS). Indeed, the aim of the experiments carried out was to get low DS, just enough to reach sufficient hydrophobicity and to have soft reaction conditions. Several starch formiates were successfully synthesized with a DS varying from 0,35 to 0,79.
It is important to better understand the mechanisms of destructuration of starch by formic acid. Indeed, in order to optimize the process of biodegradable materials, a given level of destructuration of starch is required. Thus, rheological properties of the formiates were determined to emphasize formic acid influence during the gelatinisation of starch. It was observed that formic acid acts both as a reactant and as a denaturating agent. Indeed, above the gelatinisation temperature, the gel structure of starch is destroyed when formic acid is added. Destructuration was followed by optical microscopy too. This experiment provided information on kinetics of gelatinisation.
Further experiments will be carried out so as to better characterise the level of destructuration of starch formiates synthesized (light scattering, AFM).
Poly(ester-amidE)S–pREPARATION AND PROPERTIES
D. Chromcováa, A. Bernáškováa, R. Lagaa, J. Brožeka, J. Rodaa,
V. ŠAŠEKb, J. NÁHLÍKc
aDepartment of Polymers, cDepartment of Solid State Engineering, Institute of Chemical Technology, Technická 5, CZ-166 28 Prague 6
bInstitute of Microbiology, Academy of Sciences of the Czech Republic, CZ-142 20 Prague 4, Czech Republic, e-mail: Daniela.Chromcova seznam.cz
Aliphatic polyesters such as poly(e -caprolactone) (PCLO) are an important class of biodegradable synthetic polymers. Relatively low melting points restrict their application possibilities. On the other hand, aliphatic polyamides such as poly(e -caprolactam) are known for their inertness to biodegradation. Poly(ester-amide)s have been reported to be biodegradable, their properties vary depending on the amount and type of amide and ester components in polymerization mixture and polymerization conditions.
Two procedures have been used for the preparation of
poly(ester-amide)s – by anionic copolymerization of e -caprolactam (CL) with e
-caprolactone (CLO) or
d -valerolactone and polymerization of
CL in the presence of PCLO. Thus prepared materials were
characterized by incorporation of ester units in resulting
copolymer, thermal methods (TGA, DSC and DMA). Mechanical
properties were also determined.
Poly(ester-amide) foils for biodegradation tests were prepared by melt pressing at temperature 20°C above their melting temperatures or prepared from a solution in formic acid. Two degradation tests were carried out – hydrolysis in buffer solution (pH = 7) at 60°C and treatment with six species of ligninolytic fungi in two types of culture media. Depending on poly(ester-amide) composition the changes in weight of samples after degradation and in molecular structure indicated their sensitivity to abiotic hydrolysis. The SEM of surface structure of foils after fungal treatment revealed that some fungi, especially species Irpex lacteus, Trametes versicolor and Phanerochaete chrysosporium, significantly eroded the foils.
The research was supported by grant No. 203/03/0508 of the Grant Agency of the Czech Republic.
NEW ENZYMATIC REACTIONS FOR THE DEGRADATION OF POLYURETHANES
T. KIMURAa, T. WATANABEb
aBio Research Laboratory, Future Project Division, Toyota Motor Corporation. 1, Toyota-cho, Toyota, Aichi 471-8572, Japan
bLaboratory of Biomass Conversion, Wood Research Institute, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan
Polyurethanes are an important and versatile class of man-made polymers used in a wide variety of products including paints and coatings, padding and thermal insulating materials. These polymers are synthesized from polyols and polyisocianates, and are classified into two types, polyester polyurethanes, and polyether polyurethanes.
Despite its xenobiotic origins, polyester polyurethane has been found to be susceptible to biodegradation by naturally occurring microorganisms. Enzymatic attack on polyester polyurethanes could be contributed by hydrolases such as ureases, proteases and esterases. However, very little actually is known about the enzymatic degradation of polyether polyuretanes.
Prompted by the studies about the mechanisms of lignin degradation by fungi, we found and developed the new enzymatic reaction system for the degradation of polyuretanes, especially polyeter polyurethanes. Using this reaction, the polyether polyurethanes were almost completely degraded for 2 weeks. The degradation mechamism will be also discussed.
(1) Polyurethanes are a class of plastics which are widely used as raw materials in various industries. a
Polyurethanes are an important and versatile class of man-made polymers used in a wide variety of products including paints and coatings, padding and thermal insulating materials. b
(2) Polyurethanes are synthesized from polyols and polyisocianates, and are classified into two types, polyester polyurethanes, and polyether polyurethanes. a
This polymer represents the first industrially important and commercially useful culmination of an old line of organic research (namely, isocyanates). B
Polyurethane is a polymer in which the repeating unit is a urethane linkage. B
The urethane linkage results most readily through the reaction of the isocyanate function with an alcohol group. B
Urethanes are esters or amide esters of carbonic acid. B
However, the chemical term urethane is devoid of meaning and is better described as ethyl carbamate. b
(3) Polyester polyurethanes are considered to be comparatively susceptible to microbial degradation. a
Despite its xenobiotic origins, polyurethane has been found to be susceptible to biodegradation by naturally occuring microorganisms. B
Enzymatic attack on polyurethanes could be contributed by hydrolases such as ureases, proteases and esterases. b
Recycling of waste plastics has become very important because of shortages in raw materials for the synthesis of plastics. A
Due to the incompatibility of most polymers and the econormous difficulties encountered in segregating polymeric articles of different chemical composition found in plastic waste, complete recycling is difficult and limited to special cases.
DeVULCANIZATION of POLYISOPRENE RUBBERS by WOOD ROT Fungi
S. SATOa, Y. HONDAa, K. Muraokab, T. WATANABEa
aLaboratory of Biomass Conversion, Wood Research Institute, Kyoto University, Gokasho Uji Kyoto 611-0011, Japan, e-mail: ssato kuwri.kyoto-u.ac.jp.
bSumitomo Rubber Industries, Ltd. Kobe 651-0071, Japan
cis-1,4-Polyisoprene, a main constituent of natural rubber is synthesized by more than 2000 species of plants. As an alternative to the biological production, cis-1,4-polyisoprene is also synthesized chemically to obtain the so called isoprene rubbers. These raw materials are converted to elastic rubber products by the process of vulcanization that leads to cross-linkages between elastomer chains. Vulcanization gives the rubber elasticity and recalcitrance to chemical and biological degradation. In the present research, we focused on white rot fungi. These fungi are able to degrade lignin in wood by extracellular free radical processes mediated by oxidative enzymes and transition metals. Since lignin is a recalcitrant three-dimensional polymer, we applied white rot fungi to the degradation of vulcanized rubbers.
Vulcanized natural rubber sheets with 1 mm thickness were treated with two white rot fungi, Ceriporiopsis subvermispora and Dichomitus squalens. Five of the natural rubber sheets were put onto 10 g of beech wood chips containing 10 ml of nutrients composed of 0.7% glucose and 0.7% corn steep liquor. Fungal agar pellets pre-incubated for 1 week were inoculated into the flasks and the cultivation was carried out statically at 28 ºC with 70% humidity from 0 to 250 days. Total sulfur content of the rubber decreased by 54% after cultivation of C. subvermispora for 200 days. ESCA demonstrated increase in S-O bonds and decrease in S-C bonds. Swelling ratio of the rubber in toluene increased by the cultivation. 13C-NMR demonstrated that C. subvermispora decreased number of monosulfide bonds than that of polysulfide bonds. These results indicate that C. subvermispora decreased sulfur cross-linkages due to the scission of sulfur-carbon bonds. In contrast, D. squalens caused no significant changes of the sulfide bonds. The oxidative scission of sulfide bonds with a white rot fungus, C. subvermispora can be proposed as a new biological devulcanization system for rubber recycling.
AROMATIC-ALIPHATIC COPOLYESTERS BASED ON RECLAIM POLY(ETHYLENE TEREPHTHALATE) AND THEIR BIOLOGICAL DEGRADABILITY
I. PROKOPOVÁa, V. ŠAŠEKb, J. NÁHLÍKc, E. VLČKOVÁa, L.
PLCHOTOVÁa,
J. SKOLILa
aDepartment of Polymers and cDepartment of Solid State Engeneering, Institute of Chemical Technology, Technická 5, CZ-166 28 Praha 6, Czech Republic, bInstitute of Microbiology, Academy of Science of the Czech Republic, Vídeňská 1082, CZ-142 20 Praha 4, Czech Republic
Aromatic-aliphatic copolyesters are an interesting type of polymers which can join good material properties of aromatic polyesters and the sensitivity to biodegradation of the aliphatic polyesters. In the last decade considerable interest has been focused on the polycondensation of diols, aliphatic and aromatic dicarboxylic acids or their esters on one hand and on transesterification of aromatic and aliphatic polyesters on the other one.
An alternative to the mentioned syntheses of aromatic-aliphatic copolyesters is the chemical modification of poly(ethylene terephthalate) (PETP) with lactic acid (LA) or with 1,2-ethanediol and adipic acid (AA). Material from used PETP bottles has been employed for this chemical modification. This route for preparation of copolyesters with enhanced sensitivity to biodegradation is at the same time one possible way for reutilization of PETP scrap.
The biodegradability of synthetized copolyesters based on reclaim PETP was tested both by composting and fungal treatment. Copolyester films were treated with six species of ligninolytic fungi in two different culture media for 35 days at 24°C. According to SEM analysis the copolyester PETP/ED/AA films are most distored due to effect of ligninolytic enzymatic complex of the fungi Pleurotus ostraetus, strain 670/93 and Phanerochaete chrysosporium, strain ME 446. The surface of PETP/LA films was unmistakable damaged with the fungus Irpex lacteus, strain 617/93. The erosion of aromatic-aliphatic copolyester films composted for 42 days buried in the compost was more pronounced than after fungal treatment. Composted samples are characterized by strong changes in their molecular structure compare to the original copolyester. PETP films remain after composting under identical conditions unchanged.
This work was supported by grant No. 203/03/0508 of the Grant Agency of the Czech Republic.
STUDY OF BIODEGRADABILITY OF POLYMERS WITH AUTOMATIC ANALYSER
M. JULINOVÁa, J. HOFFMANNa, V.SEDLAŘÍKa, D. BAKOŠb
aTomas Bata University in Zlín, Faculty of Technology, Department of Environmental Technology and Chemistry, nám. TGM 275, 762 72 Zlín, Czech Republic
bSlovak University of Technology, Faculty of Chemical and Food Technology, Department of Plastics and Rubber, Radlinského 9, 812 37 Bratislava, Slovakia
The paper presents potential use of automatic analyser Micro Oxymax (Columbus, Ohio, U.S.A.) for evaluating biological degradability of polymers. Conveniency of the given analyser was assessed on biodegradation of particular components and mixed films containing PVAL, modified protein hydrolysate (Hykol), natural untreated starch, plasticised starch with added caprolactam and/or their combinations. Biodegradability of tested samples was studied on an aerobic aqueous environment containing a mixed microbial culture in the form of non-adapted or PVAL-adapted activated sludge from the municipal waste-water treatment plant. Course of biodegradation was evaluated by IR-spectrophotometric determination of oxygen consumption and of CO2 production by means of the mentioned automatic analyser. A further effect under study was that of suspension stirring mode (rotating, swinging/rocking ”orbital”) on resultant CO2 production and oxygen consumprion. This fully ”automated” process is an alternative method of assessing biodegradability and may complement existing standard tests. The aim of work was confronting results of these tests with results obtained through ”classic” standard respiratory procedures (measuring oxygen demand or carbon dioxide production). It was found through standard tests in another work that a ”stepwise” degradation course occurs with mixed films when non-adapted inoculum is applied, corresponding to gradual breakdown of individual components in the order of their biological degradability. Also noted was the significant effect of inoculum adaptation on the length of initial lag phase.
In analogous tests with analyser Micro Oxymax it was found that results of individual tests were in accord and the analyser, price not being taken into account, could prove to be a very good alternative to existing standard tests.
This work was supported by Research Project of the Ministry of Youth, Education and Sports of the Czech Republic No. MSM 281100002.
BIODEGRADABLE POLYESTER NANOCOMPOSITES:
PREPARATION AND MECHANICAL PROPERTIES
D. KUBIES1,2, T. ZAPOROZHETS1,3, R. PUFFR1, J. KOTEK1, J. BALDRIAN1, J. KOVÁŘOVÁ1, F. RYPÁČEK1
1 Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky Sq.2, Prague 162 06, Czech Republic;
2 Center for Cell Therapy and Tissue Repair, Charles University, 2nd Faculty of Medicine, Institute of Neuroscience, V Úvalu 84, 150 18, Prague 5
3 Lviv Polytecnic National University, st. S.Bandery 12, Lviv 79013, Ukraine
In contrast to pure polymer materials, layered-silicate polymer nanocomposites exhibit improved mechanical properties as well as enhanced barrier properties and resistance to fire and ignition. One of the interesting areas is their application in the biomaterials field, though still a little disregarded.
Biodegradable polyesters such as poly(e -caprolactone) (PCL) and poly(L-lactide) (PLLA) are widely used and studied for medical use. Potential application of their silicate nanocomposites is especially in surgical implant field, where the silicate should affect not only the mechanical properties of the polymer matrix but also the polymer degradation behavior. The degradation of the PCL or the PLLA bulk material is often modulated by addition of plasticizing agents (oligomers, low-molecular-weight PCL or PLLA) which, unfortunately, leads to decrease in the stiffness.
To eliminate this effect, high-silicate content nanocomposite masterbatches of PCL and organically modified montmorillonite Cloisite 30B were prepared by in situ ring-opening polymerization of lactones. Since the initiating species of the e -caprolactone polymerization were the end hydroxy groups of alkylammonium cations anchored on the surface of silicate sheets, tethered polyester nanocomposites were prepared. Morphology of the masterbatch, from intercalated to exfoliated, was varied by the composition of the polymerization mixture and depended on the molecular weight of the formed polymer.
The PCL and/or PLA nanocomposites were prepared by melt blending of high- molecular-weight homopolymers PCL and PLLA and the nanocomposite masterbatch. An addition of the PCL/layered silicate masterbatch into the PCL matrix increases both stiffness and yield stress. Both characteristics increase with increasing inorganic content and increasing degree of intercalation/exfoliation. Mechanical properties of the materials with PLLA matrix are affected by the presence of low-molecular-weight PCL, which decreases stiffness and tensile strength.
The selected samples with interesting properties are under in vitro degradation study.
Acknowledgment:
Financial support of this research by the Grant Agency of AS CR grant No. KJB 4050309 is gratefully acknowledged.
Crosslinking of Polybutadiene. Correlation between solid-state 1H NMR, Thermoporosimetry, Densimetry and Crystallinity Measurements
J.F. PILICHOWSKIa, T. LIPTAJb, M. MORELa, M. BABAa
a Laboratoire de Photochimie, UMR 6505 du CNRS, Université B. Pascal, Les Cézeaux, 63177 Aubière Cedex, France. b Central Laboratory, Slovak University of Technology, 812 37 Bratislava, Slovak Republic
The process of polybutadiene cross-linking initiated by dicumyl peroxide was studied by various analytic techniques. 1H-NMR was used to follow the decrease of polymeric chains mobility by measurement of T2. The change in degrees of freedom leads to a diminution of the ability of elastomer to crystallize. DSC measurements were then used to quantify the residual crystallinity of the polymer. The solubility of elastomeric material depends on the size of its macromolecular chains. Cross-linking increases considerably the length of these chains and it was possible, by measurement of the density of the solution of the extractable part of the elastomer, to follow the growing of the polymeric network up to its gel point. Beyond the gel point, the elastomer becomes completely insoluble but it is possible to study the swollen gel properties. The swelling solvent, cyclohexane in our case, can be used as a textural probe giving relevant details about the size distribution of the polymeric segments between cross-link nodes. By thermoporosimetry and using the solid-solid thermal transition of cyclohexane, it was possible to follow the decreasing of the distance between the cross-linking nodes as well as the distribution of the network.
The main conclusions of this work are : i) good correlation can be observed between the results of the analyticals methods used; ii) moreover, NMR spectroscopy is suitable to quantify the level of cross-linking during ageing.
These preliminary results encourage us to plan to apply the same approach to study the cross-linking provoked by the thermo or photo-aging of elastomers.
STUDY OF INDUCTION PERIODS of polymer oxidation
by an integral isoconversional method
P. ŠIMON, Z. CIBULKOVÁ, M. FRATRIČOVÁ
Department of Physical Chemistry, Faculty of Chemical and Food Technology,
Slovak University of Technology, Radlinského 9, SK-812 37 Bratislava, Slovak Republic
Many processes exhibit the induction period, i.e. the stage where seemingly no chemical reaction takes place. In some cases, accurate determination of the induction period is of utmost importance for the safety and quality management. In this paper, an integral isoconversional method is proposed for obtaining the kinetic parameters of induction periods from the onset temperatures of nonisothermal DSC runs with a linear increase of temperature. Dependence of the induction period on temperature can be expressed by an Arrhenius-like relationship
![]() (1)
(1)
where A and B are constants and T is the absolute temperature. In the case of linear increase of temperature, the parameters A and B in eqn.(1) can be obtained by minimizing the sum of squares between experimental oxidation onset temperatures and the temperatures calculated from eq.(2)
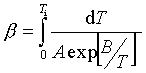 (2)
(2)
where Ti is the temperature of the end of induction period, i.e. the onset temperature of the main oxidation process and b stands for the coefficient of temperature increase (scan) [1].
The method is applied to the study of thermooxidation of four polymers, i.e., polyethylene, polypropylene (polyolefines), polyisoprene and polyurethane. The main features of the thermooxidation of the polymers mentioned are discussed. In all cases, the sources of systematic errors are analyzed. It is shown that the nonisothermal DSC method gives the kinetic parameters describing the induction periods more reliably than the related isothermal methods.
REFERENCES
[1] Šimon P. & Kolman Ľ., Journal of Thermal Analysis and Calorimetry 64 (2001) 813-820.
Radiation-induced embrittlement of PTFE
L. Audouin, B. Fayolle, J. Verdu
Laboratoire de Transformation et de Vieillissement des Polymères, Ecole Nationale Supérieure des Arts et Métiers, 151 Boulevard de l‘Hôpital, 75013 Paris, France
E-mail: LTVP paris.ensam.fr
The radiochemical degradation of polytetrafluoroethylene (PTFE) samples has been studied in air at dose rate 100 Gy/h for doses up to 5000 Gy, at ambient temperature.
The polymer degradation has been monitored by DSC, tensile testing and Essential Work of Fracture (EWF) testing. Some fractured samples have been observed by scanning electron microscopy.
The polymer undergoes a fast chain scission, its number average molar mass is divided by about 20 for a dose of 1000 Gy and tends towards a pseudo asymptotic value of ~ 20 kg mol-1 (against 6200 kg mol-1 initial value). The modulus and yield characteristics seem to be almost unaffected whereas ultimate properties undergo strong variations. The ultimate elongation R and the EWF plastic work characteristic wp first increase and then decrease. The ultimate stress decreases and tends towards a pseudo asymptotic value.
The mechanisms of radiation induced ultimate property changes are discussed.
A critical molar mass M’C governing the ductile-brittle transition of semicrystalline polymers
B. FAYOLLE, L. AUDOUIN, J. VERDU
Laboratoire de Transformation et de Vieillissement des Polymères, Ecole Nationale Supérieure des Arts et Métiers, 151 Boulevard de l‘Hôpital, 75013 Paris, France
E-mail: LTVP paris.ensam.fr
We show that semi-crystalline polymers of low polarity (PE, PP, PTFE) become brittle when their weight average molar mass MW becomes lower than a critical value M’C of about 50Me with Me being the molar mass between entanglements in the melt.
These M’C values are also found when MW changes result from thermoxidation, radioxidation or simply from synthesis showing that this ”ductile-brittle” molar mass is a polymer characteristic.
The fact that a very small number of chains play a key role in plastic deformation is tentatively explained by the fact that interlamellar ties are composed of several entanglements strands.
Degradation Kinetics of pvc mixed with additives
T. KARAYILDIRIMa, J. YANIKa, M. YUKSELb, M. SAGLAMb, H. BOCKHORNc
aDepartment of Chemistry, Faculty of Science, Ege University, 35100 Izmir,Turkey
bDepartment of Chemical Engineering, Ege University, 35100 Izmir,Turkey
cInstitute of Chemical Engineering, University of Karlsruhe, Engesserstraße 20, D-76131 Karlsruhe, Germany
In this work, the effect of some additives on the thermal degradation of PVC was investigated by thermogravimetry / mass spectrometry (TG / MS). The additives used were Red-Mud (RM), CaCO3 and dolamite which are all chlorine fixators for the pyrolysis of a PVC containing polymer mixture. In the presence of carbonates, the inflection point (Tmax) of dechlorination shifted to a higher temperature and the rate of mass loss at Tmax decreased. However in the presence of RM, FeCl3 formed and this accelerated the dechlorination of PVC, but retarded the second step of PVC decomposition. The shape of MS ion curves of the degradation products was similar to the DTG curves. RM reduced the onset temperature of HCl formation whereas carbonates depressed HCl formation. Benzene formation was suppressed by the additives. In addition, in the presence of RM, evolution of benzene and other aromatics shifted to higher temperatures.
THERMAL DEGRADATION OF UV-IRRADIATED POLY(VINYL CHLORIDE) CONTAINING METAL SALTS
H. KACZMAREK*, J. KOWALONEK, A. SIONKOWSKA
Faculty of Chemistry, Nicolaus Copernicus University, Gagarin 7, 87-100 Toruń, Poland
*E-mail: halina chem.uni.torun.pl
Poly(vinyl chloride) (PVC) is widely used thermoplastic polymer, so it is intensively investigated since many years. This polymer exhibits relatively low thermal and photochemical stability, which can be changed by its physical or chemical modifications. Inorganic compounds can catalyze or inhibit polymer decomposition under various factors. Morphology of modified sample and interactions between composition components strongly affect the course of degradation processes.
In this work the influence of small amount of CoCl2 and FeCl3 (1-5% wt) on UV-irradiated PVC was studied using thermogravimetry.
Thermal degradation of PVC is two-steps process. Dehydrochlorination with polyenes formation occurs in the first step while in the second one – decomposition of polyene structures with chain scission takes place.
It was found that thermal degradation in PVC containing CoCl2 and FeCl3 starts earlier (at significantly lower temperatures) than that in pure PVC, which indicates the destabilizing effect of both salts on polymer.
However, the decomposition of polyene sequences is somewhat hampered in the presence of FeCl3. CoCl2 has only negligible influence on the beginning of this second degradation step.
Short wavelength UV-irradiation (254 nm) caused the decrease of the initial temperature of PVC dehydrochlorination. It means that thermal stability of exposed samples is lower. The highest changes in thermal stability after UV-irradiation were observed in PVC with iron salt.
The mechanisms of competitive reactions occurring in doped PVC during UV-irradiation and heating have been discussed.
Acknowledgement
Financial support from the Polish State Committee for Scientific Research (KBN; grants No 3 T09B 088 18 and nr 4 T09A 16622) is gratefully acknowledged.
LOW-TEMPERATURE DEGRADATION OF PLASTICIZED PVC FORMULATIONS CROSSLINKED WITH γ RADIATION
R. BENAVIDES1, A.O. CASTAÑEDA-FACIO1, M.E. MARTÍNEZ-PARDO2, M. SÁNCHEZ-ADAME1.
1Centro de Investigación en Química Aplicada, Blvd.. Enrique Reyna H. 140, Saltillo, Coahuila, 25294, México. E-mail: robertob polimex.ciqa.mx
2Instituto Nacional de Investigaciones Nucleares, Apdo. postal 18-1027, Col. Escandón, 11801, México D.F., México.
Cable formulations of plasticized PVC, stabilized with lead, Ca/Zn (2:1) and Ca/Zn (1:1) and doped with and without a crosslinking agent (TMPTMA), were exposed to gamma radiation at doses of 50, 75 and 100 kGy, under either air or argon atmospheres. Their gel percentage by extraction and Young´s modulus by TMA were monitored to evaluate the performance of the non-toxic materials. The irradiated materials were also degraded at 120 C during 48 hours and their colour changes and modulii obtained.
The crosslinking agent makes high differences in gel percentage among formulations. When TMPTMA is included, values of 50-70 % gel are obtained from the lowest radiation dose of 50 kGy, while without it they remain below 10 %. The exception comes with the formulation stabilized with Ca/Zn (1:1), where the gel % has no trend. Such effect is caused by the high amount of zinc stearate.
The evaluation of modulii show higher values for the formulations doped with the TMPTMA and a very distinctive increase in the values at the dose of 75 kGy, when irradiated in inert atmosphere (Fig. 1); while irradiated in air the effect is not that clear, since degradation is more important than crosslinking (Fig.2). It is interesting that the formulation of Ca/Zn (2:1) has higher modulus than the lead formulation at 75 kGy (in Ar)
When the formulations were degraded in the oven at 120 C during 48 hours, colouration of the samples varied from light yellow to dark brown, with a general tendency to have less colour for the 75 kGy dose, even at the longer degradation time. The modulii for all of them remained unchanged during such degradation time. It is on the way a study on the environmental aging to evaluate the migration of plasticiser and the lost of properties.
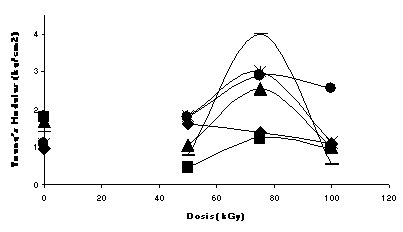
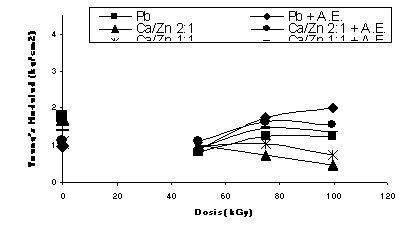
Fig.1 Non-degraded formulations irradiated in Argon Fig.2 Non-degraded formulations irradiated in air
THERMAL DEGRADATION OF EPOXY-RESIN PVC PLASTISOL BLENDS
A. IANNONI1, L. TORRE1, A. JIMÉNEZ2, J.M. KENNY1
1University of Perugia at Terni. Loc. Pentima Bassa, 21, 05100, Terni, Italia.
2University of Alicante. Department of Analytical Chemistry. PO Box 99, 03080, Alicante. Spain.
Epoxy resins are used worldwide on a large scale for adhesive, lamination, coating and casting applications and so forth. Many of these uses are based on their high rigidity which, in general, is associated with high mechanical properties. However, some of the main drawbacks of the properties of epoxy resins are their high fragility and poor impact properties, which limits their application. Therefore, the addition of another component to the resin, which improves the flexibility of the blend, would be a promising possibility to widen their application. PVC plastisols are based on the addition of plasticizers at high concentrations (20-30% or even higher) to PVC resins. The addition of plasticizers to a PVC formulation decreases many of the mechanical properties of a PVC product (hardness, tensile strength, modulus, etc.), however, low-temperature flexibility, elongation and the ease of processing are all improved. The main goal of this work was to study the thermal degradation of these epoxy resin – PVC plastisol blends with a determination of the main apparent kinetic parameters. DGEBA was selected as the epoxy resin and DETA as the curing agent for this study. A commercial PVC resin (Vestolit- 7021, Huls, Germany) mixed with di-ethyl hexyl phthalate (DEHP) was used for the preparation of the plastisols. DSC studies showed that the ideal stochiometry for the resin was 4:1 as H values were higher for this combination. After addition of the PVC plastisol, there was a clear decrease in H, which means that no chemical interaction between the components was observed. In addition, Tg values decreased with higher plastisol concentrations. Dynamic TGA studies showed that the maximum degradation temperatures for blends were clearly lower (70°C lower for blends with 30% plastisol compared to pure epoxy resin). This indicates that the thermal degradation of the blends starts at lower temperatures and therefore, some kind of stabilizer is necessary in order to permit processing and use at high temperatures. Isothermal tests were also carried out in order to confirm this behaviour. We observed that at temperatures between 270 and 300°C, the degradation of the blends was much faster than in the case of pure epoxies. Apparent kinetic parameters were also determined using the Friedman isoconversion method. The apparent activation energy was also calculated and some differences in the resulting values indicated this loss of stability.
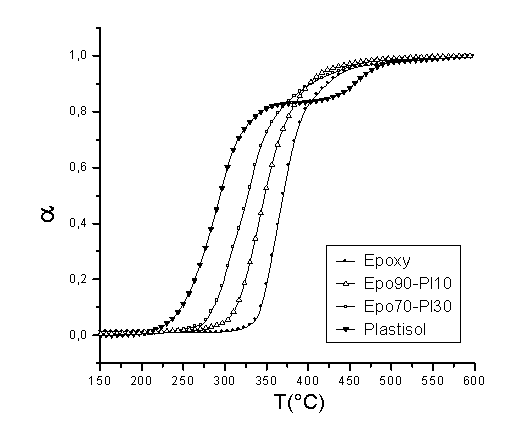
Figure 1. Tg for several epoxy-plastisol blends
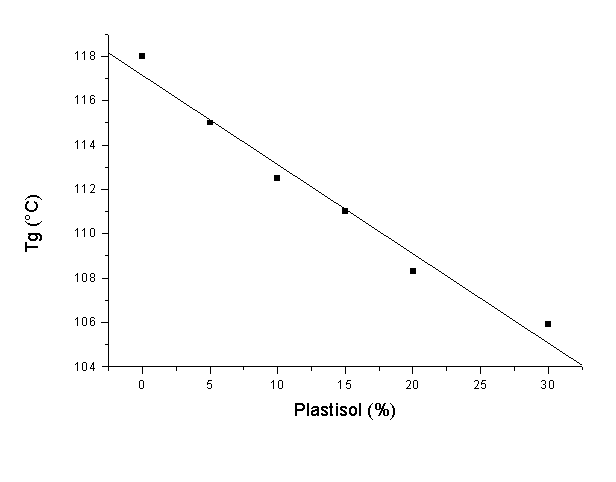
Figure 2. Dynamic TGA of several epoxy-plastisol blends
Aknowledgements. Stefano Bracchini and Fernando Magallanes carried out some of the experimental work.
References
C.L. Chiang, C.C.M. Ma, F.Y. Wang, H.C. Kuan, Eur.Polym. J. 39 (2003) 825–830
V.J.R.R. Pita a, E.E.M. Sampaio b, E.E.C. Monteiro, Polymer Testing, 21 (2002), 545-550
THERMAL DEGRADATION OF CROWN-ETHER AND
CALIX[4]PYRROLE-CONTAINING POLYACRYLAMIDE-BASED HYDROGELS
A. JIMÉNEZ, A.J.F.N. SOBRAL*, A.J.M. VALENTE*, V.M.M. LOBO*
Department of Analytical Chemistry, University of Alicante, PO Box 99, 03080 Alicante, Spain; alfjimenez ua.es
* Department of Chemistry, University of Coimbra, 3004-535 Coimbra, Portugal
The past two decades have seen a growing interest in the use of neutral chelating agents to achieve ion permselectivity in membranes.1-5 Membranes containing these neutral ion selective ligands can be exploited in separation processes or as the sensing component in ion-selective electrodes. Much work has been done incorporating neutral chelating agents, such as crown ethers, by physical immobilisation, in hydrophobic membranes. In the last years some work has been done in the study of physical immobilisation of crown-ethers in the in water-swollen polymers.5,6 Our main goal is to synthesise and characterise hydrogels containing chelant agents by physical and chemical immobilization as well as their behaviour at high temperatures. In this communication thermal degradation of 4’-(3-acryly)-benzo-15-crown-5 (ABC) and calyx[4]pyrrole containing polyacrylamide was studied.
Dynamic and isothermal thermogravimetric analysis (TGA) was used for the study of thermal degradation of these co-polymers. As they are highly hydrated materials, low heating rates at temperatures below 100ºC were used in order to eliminate all the non-freezing water and to obtain the base material. Furthermore, a complete dynamic run was carried out and kinetic parameters were calculated by application of classic models, such as Friedman. First results showed that more than 70% in weight of the polymer was non-freezing water and the rest showed a continuous loss of weight up to 400 ºC. Apparent activation energies were also calculated and those results permitted a comparison for the thermal stability of samples with different ABC and calyx[4]pyrrole amounts.
In addition, the thermal degradation of these polymers and polyacrylamide-based hydrogels was compared and differences were observed at high temperatures.
References
1. E. Shchori, J. Jagur-Grodzhishi, J. Appl. Polym. Sci. 1976, 20:773
2. W. Simon, E. Pretsh, D. Ammann, W.E. Morf, M. Guggi, R. Bissig, M. Kessler, Pure Appl. Chem. 1975, 44:613.
3. A.K. Convington (Ed.). Ion-selective electrode methodology, I. Florida:CRC Press, 1979.
4. J.D. Lamb, J.J. Christensen, S.R. Izatt, K. Bedke, M.S. Astin, R.M. Izatt, J. Am. Chem. Soc., 1980; 102:3399
5. C.J. Hamilton, S.M. Murphy, B.J. Tighe Polymer, 2000, 41:3651.
6. C.F. Reusch, E.L. Cussler. AIChE J. 1973,19:736.
Acknowledgement
We would like to thank Projecto Luso-Espanhol (E-2/02), FCT (Sapiens POCTI/QUI/42536/2001) for financial support.
polyamide - montmorillonite Nanocomposites
R. Puffra, J. Brožekb, J. Špátováb, J. Baldriana
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovský Sq. 2, CZ-162 06 Prague 6, e-mail: puffr imc.cas.cz
bDepartment of Polymers, Institute of Chemical
Technology, Technická 5,
CZ-166 28 Prague 6, e-mail: Jiri.Brozek vscht.cz
Polyamide nanocomposites were synthesized by polymerization of
e -caprolactam, h
-capryllactam, w -laurolactam and N-methyl-w -laurolactam in the presence of various
amounts of a layered silicate - montmorillonite - intercalated
with w -amino lauric acid
hydrochloride. Swellings of the intercalate in the lactams were
evaluated by XRD analysis. The interlayer spacing was further
increased, most in e -caprolactam and
least in N-methyl-w
-laurolactam.
Polymerizations were initiated with the respective amino acids, in the case of e -caprolactam anionic initiators have also been used. The presence of the intercalate in polymerization mixtures accelerated otherwise slow hydrolytic polymerizations.
Poly(e -caprolactam) nanocomposites were also prepared by melt-blending Ultramid with the intercalate using a 5-cm3 twin-screw extruder.
Missing diffraction peaks of silica layers in the WAXS diffractograms of the composites, with an exemption of those prepared by anionic polymerization, indicated that interlayer spacings exceeded 6 nm or the layers were entirely disordered.
The crystalline structure of polyamide phase evaluated by WAXS revealed dependence of the -and g -structure content on thermal history of the composite (cooling rate) and presence of silicate.
Thermal properties of the intercalates and nanocomposites were evaluated by DSC and TGA methods.
The research was supported by the Ministry of Education, Youth and Sports of the Czech Republic, project No. MSM 223100002.
Electrical Cables in Czech Nuclear Power Plants — Simulation of their Degradation
V. HNÁT, V. PLAČEK, B. BARTONÍČEK
Department of Radiation Chemistry and Environmental Qualification
Nuclear Research Institute Řež, 25068 Řež, Czech Republic
hna nri.cz; pla nri.cz; bob nri.cz
Instrumentation and control cables are the most important ones from the point of view of nuclear power plant (NPP) safety. They have to be functional not only under the normal operation condition but also under all design accident events (especially during LOCA — Loss-of-Coolant Accident).
Possible mechanisms of cable degradation are listed. The reasons why the more than 100-times acceleration used for the simulation of their normal operation ageing cannot give a reliable forecast of their lifetime are specified. (It is due to the structural and mechanical changes of cable insulating materials over the years of ageing. These changes depend on the limited rate of oxygen diffusion at very low dose rates at real NPP operation which lead to the condition of homogeneous radiooxidation and so to the scission of macromolecular chains.)
The way of simulation of harsh environmental attack during the LOCA (simultaneous action of radiation, of steam at enhanced temperature and pressure, and of chemical spray) and the way to verify the cable functionality under such condition are also described.
Further, the approach to the management of cable ageing for Czech NPPs based on the programme of on-going qualification of cables is outlined. That consists in:
AGEING OF POLYANILINE FILMS RELATED TO TEMPERATURE AND HUMIDITY
E. TOBOLKOVÁa , J. PROKEŠa , I. KŘIVKAa , M. TRCHOVÁb , J. STEJSKALb
aCharles University Prague, Faculty of Mathematics and Physics, Ke Karlovu 5, CZ-121 16 Prague 2, Czech Republic
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Prague 6, Czech Republic
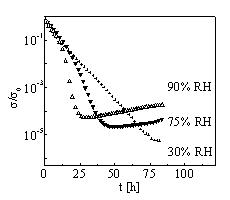 DC
conductivity of polyaniline hydrochloride films [1] was
investigated in an atmosphere at elevated temperature and defined
air humidity. Conducting layers prepared on glass supports were
placed to a humidity chamber, where the atmosphere of constant
relative humidity (RH) ranging from 30% to 90% and a temperature
elevated to 80°C were held for 85 hours. Time dependence of dc
conductivity s (t) was measured
in situ (FIG.1 – s 0
is the conductivity measured at 25°C and 50% RH). The initial
decrease of s (t) to its
minimum represents a response to the temperature step and can be
explained in terms of degradation of granular structure [2]. The
next shape of s (t) strongly
depends on RH and points to presence of a process, in which
molecules of absorbed water take part and which can temporarily
increase the conductivity. This corresponds well to the behaviour
observed before for ageing of the PANI hydrochloride films at
90°C [3]. A simple model taking this increase of conductivity
into account was proposed in [3] and the present results have
been discussed in this way. The molecular nature of the process
itself remains still under discussion. FTIR study was used to
examine the degree of de- protonation of the degraded films.
DC
conductivity of polyaniline hydrochloride films [1] was
investigated in an atmosphere at elevated temperature and defined
air humidity. Conducting layers prepared on glass supports were
placed to a humidity chamber, where the atmosphere of constant
relative humidity (RH) ranging from 30% to 90% and a temperature
elevated to 80°C were held for 85 hours. Time dependence of dc
conductivity s (t) was measured
in situ (FIG.1 – s 0
is the conductivity measured at 25°C and 50% RH). The initial
decrease of s (t) to its
minimum represents a response to the temperature step and can be
explained in terms of degradation of granular structure [2]. The
next shape of s (t) strongly
depends on RH and points to presence of a process, in which
molecules of absorbed water take part and which can temporarily
increase the conductivity. This corresponds well to the behaviour
observed before for ageing of the PANI hydrochloride films at
90°C [3]. A simple model taking this increase of conductivity
into account was proposed in [3] and the present results have
been discussed in this way. The molecular nature of the process
itself remains still under discussion. FTIR study was used to
examine the degree of de- protonation of the degraded films.
Acknowledgement. The research was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (A4050313) and the Grant Agency of the Charles University (188/2003).
[1] Stejskal J, Sapurina I, Prokeš J, Zemek J, Synth. Met. 105 (1999)195.
[2] Wolter A, Rannou P, Travers J P, Giles B, Djurado D, Phys. Rev. B 58 (1998) 7637.
[3] Tobolková E, Prokeš J, Křivka I, Trchová M, Stejskal J, Macromol. Symp. in press.
AC CONDUCTIVITY AND CHARGE TRANSPORT IN AGED POLYANILINE
O. STARYKOVa, J. PROKEŠa, I. KŘIVKAa, J. STEJSKALb
aCharles University Prague, Faculty of Mathematics and Physics, Ke Karlovu 5, CZ-121 16 Prague 2, Czech Republic
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Prague 6, Czech Republic
Polyaniline hydrochloride, prepared at two different
temperatures (![]() °C and
°C and
![]() °C), was aged as
pellets in the thermal chamber, as described in [1]. Samples were
exposed to several temperature cycles between room temperature
and 146°C or 179°C. AC conductivity of obtained materials was
measured in frequency range 20 Hz–5 MHz and temperature range
100–300 K.
°C), was aged as
pellets in the thermal chamber, as described in [1]. Samples were
exposed to several temperature cycles between room temperature
and 146°C or 179°C. AC conductivity of obtained materials was
measured in frequency range 20 Hz–5 MHz and temperature range
100–300 K.
X-ray analysis showed that polyaniline prepared at –50°C has a higher degree of crystallinity than that prepared at +25°C [1]. The best fit of frequency dependence of AC conductivity was made for the model described by Bianchi [2]. It confirms that aged polyaniline can be represented by conducting islands distributed in the amorphous disordered matrix [3]. Temperature of polymerisation plays important role in sample formation and later in ageing process. Samples with higher crystallinity possess slower ageing, as a result of a higher volume fraction and conductivity of conducting islands, and lower permittivity and charge-hopping length in amorphous matrix. The ageing is most probably caused by the destruction of conducting-islands borders, resulting in the decrease of their size.
[1] J. Prokeš, M. Trchová, D. Hlavatá, J. Stejskal, Polym. Deg. Stab. 78, 393 (2002).
[2] R.F. Bianchi, G.F.L. Ferreira, C.M. Lepienski, R.M. Faria, J. Chem. Phys. 110, 4602 (1999).
[3] B. Sixou, N. Mermilliod, J.P. Travers, Phys. Rev. B 53, 4509 (1996).
THERMAL STABILITY OF POLYANILINE AND POLYPYRROLE PREPARED IN THE PRESENCE OF SURFACTANTS
M. OMASTOVÁa, J. STEJSKALb, J. PROKEŠc, M. TRCHOVÁb, J. KOVÁŘOVÁb
aPolymer Institute, Slovak Academy of Sciences, Dúbravská cesta 9, 842 36 Bratislava, Slovakia, E-mail: upolmaom savba.sk
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, 162 06 Prague 6, Czech Republic
cCharles University Prague, Faculty of Mathematics and Physics, 121 16 Prague 2, Czech Republic
Surfactants have been used as additives in the polymerization of aniline and pyrrole for two reasons: (1) to affect the locus of polymerization by using the emulsion or inverse emulsion pathways, and thus to modify the molecular and supramolecular structure of the resulting polymers, and (2) to improve the properties of the polymers with respect to conductivity, stability, solubility in organic solvents, and processibility.
Polyaniline and polypyrrole have been prepared by chemical oxidative polymerizations of the corresponding monomers in an aqueous medium containing an anionic surfactant – sodium bis(2-ethylhexyl) sulfosuccinate, dodecylbenzenesulfonic acid and its sodium salt, and sodium dodecyl sulfate. Determination of polymerization yield and density, elemental analysis, and FTIR spectroscopy prove that the anionic surfactants become incorporated into the conducting polymers. The similar inclusion of a cationic surfactant into conducting polymers was not observed. While the conductivity of polypyrrole became enhanced after the introduction of an anionic surfactant, the changes in the conductivity of polyaniline were marginal [1].
The thermal stability of conductivity at 175 °C was significantly higher for polyaniline than for polypyrrole. During about one day, polyaniline conductivity was reduced by 1–2 orders of magnitude, while 4–7 orders reduction was observed for polypyrrole. The electrical stability of polyaniline was better than that of polypyrrole. The presence of a surfactant improved the stability of conductivity of polypyrrole but reduced the electrical stability of polyaniline.
[1] Stejskal J., Omastová M., Fedorova S., Prokeš J., Trchová M.: Polymer 44 (2002) 1353.
Acknowledgement. The research was supported by the Grant Agency for Science of the Slovak Academy of Sciences (GAV-2/1060/21).
OZONE DEGRADATION OF POLYPYRROLE
F. CATALDOa, M. OMASTOVÁb, J. PIONTECKc
a Soc. Lupi Chemical Research Institute, Via Casilina 1626/A, 00133 Rome, Italy
b Polymer Institute, Slovak Academy of Sciences, Dúbravská cesta 9,
842 36 Bratislava, Slovakia, E-mail: upolmaom savba.sk
c Institute of Polymer Research, Hohe Strasse 6, 01005 Dresden, Germany
Polypyrrole (PPy) can be prepared by electrochemical or chemical oxidation of pyrrole in various organic solvents and in aqueous media. In chemical oxidative polymerization, many oxidizing agents have been used, i.e. iron (III) chloride or sulfate, and ammonium peroxydisulfate. The preparation conditions and the presence of various additives influence the properties of the conducting polymer.
Two different samples of polypyrrole have been studied. PPy-Cl prepared by chemical oxidation of pyrrole with FeCl3 and PPy-Cl-DBSNa prepared using the same oxidant and the anionic surfactant, sodium dodecylbenzenesulfonate (DBSNa).
IR spectroscopy and elemental analysis proved that the surfactant was incorporated into the PPy chain similarly as the doping anion. This results in a more compact morphology and reduced sizes of the PPy globules as shown by scanning electron microscopy study.
PPy has been treated with ozone in a fixed bed reactor. It has been shown by weight increase as function of time, by FT-IR spectroscopy, and by electronic spectroscopy that the sample PPy-Cl is much more stable toward ozone attack in comparison to the sample PPy-Cl-DBSNa containing the surfactant. The reactivity of polypyrrole with ozone is easily detectable by different techniques. The ozonized PPy samples show a significantly worse thermal stability as measured by TGA-DTA in comparison with the pristine samples demonstrating the profound thermal degradation caused by the exposure to O3. The electronic spectroscopy showed that the early stages of the ozonization reaction involve the interaction of ozone with the bipolaron and polaron defects of polypyrrole. PPy-Cl-DBSNa shows a considerably higher reactivity with ozone in the solid state. In this case the degradation reaction is fast and leads to the complete destruction of the substance as detected by IR study.
DEGRADATION OF CONDUCTING POLYMER FILMS
I. ŠEDĚNKOVÁa, E. TOBOLKOVÁa, M. TRCHOVÁb, J. STEJSKALb
aCharles University Prague, Faculty of Mathematics and Physics, 180 00 Prague 8, Czech Republic
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, 162 06 Prague 6, Czech Republic
Electrically conducting polymer films have been often investigated because of their potential use in coating applications. The chemical deposition of polyaniline (PANI) film, when the layer of polymer spontaneously forms on the surface of various materials immersed in the polymerization solution, is a promising alternative for the preparation of nanofilms on a variety of insulating and conducting, hydrophobic and hydrophilic surfaces [1].
Polyaniline films were produced by the oxidation of aniline hydrochloride (0.20 M) with ammonium peroxodisulfate (0.25 M) in dilute hydrochloric, sulfuric, and phosphoric acids. The films were polymerized in situ on silicon surfaces immersed in the reaction mixture. The thermal deprotonation and chemical stability of such films have been studied by FTIR spectroscopy. The DC conductivity was measured by the four-point method in a chamber with controlled temperature.
After annealing at 150 oC, the intensity of the broad band at wavenumbers above 2000 cm–1, characteristic of the conducting form of PANI, decreases, reflecting the decrease in the conductivity. The changes in the shape of the different spectral bands observed at the wavenumbers below 2000 cm–1 correspond to the deprotonation process in the films [2]. No additional changes of PANI structure, like the oxidation producing carbonyl groups, have been observed.
The deprotonation of the films prepared in the presence of different acids have been compared. Chloride, sulfate and phosphate counterions influence the thermal stability of films.
Acknowledgement. The research was supported by the Grant Agency of the Czech Republic (202/02/0698) and Grant Agency of the Academy of Sciences of the Czech Republic (A 4050313).
[1] Stejskal J., Sapurina I., Prokeš J., Zemek J: Synth. Met. 105 (1999) 195.
[2] Trchová M., Sapurina I., Prokeš J., Stejskal J.: Synth. Met. 135–136 (2003) 305.
EFFECT OF METAL STEARATE ANTACID COMPOSITION ON THE MELT STABILISATION PERFORMANCE OF HINDERED PHENOLIC/PHOSPHITE COMBINATIONS IN LLDPE
E. M. HOANGa, C. M. LIAUWa, N. S. ALLENa E. FONTANb, G. MARINOb
a Centre for Materials Science Research, Faculty of Science and Engineering, The Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, UK;
b Repsol YPF S. A., N-V km. 18, 28931 Móstoles, Madrid, Spain.
Metal stearates such as calcium and zinc stearates are widely used as antacid agents (i.e., acid scavengers) in stabiliser packages for polyethylenes. This study investigates the effect of both the origin of the stearine blend (i.e., vegetable or animal) and metal ion (i.e., Ca2+ or Zn2+) used in the formation of the metal stearate on the melt stabilisation performance of combinations of hindered phenolic (Irganox 1010 (I1010)) and Phosphite (Irgafos 168 (I168)) antioxidants in an LLDPE.
A multiple pass extrusion experiment was carried out at a melt temperature of 200 ° C and melt stabilisation performance was monitored by melt flow rate measurements. The level of stabiliser consumption was measured after each extruder pass via HPLC analysis of solvent extracts. Although antagonism was observed with all formulations containing metal stearates, a noticeably greater detrimental effect was apparent with metal stearates based on the vegetable derived stearine blend. The more subtle effect of the metal ion was superimposed on the latter trend and it was apparent that Zn2+ had a greater detrimental effect than Ca2+. Addition of metal stearates increased the consumption of both I1010 and I168, however, metal stearates based on the vegetable derived stearine blend gave rise to the highest stabiliser consumption.
Investigation of this seemingly surprising difference in performance stimulated analysis of the metal stearates themselves; hydroperoxide levels and other relevant data that may afford further insight in to this effect will be presented.