Main lectures: 1 2 3 4 5 6 7 8 9
Y. YAGCI
Istanbul Technical University, Department of Chemistry, Maslak, Istanbul 80626, Turkey
Photochemical radical and ionic polymerizations are widely employed in industrial applications such as surface coating and imaging technologies. Besides UV curing applications, photoinitiated polymerizations can be used in synthetic polymer chemistry. During the past decade considerable attention has been focused on controlled polymerization processes by which synthesis of polymers with well defined structures is accessible. In this presentation, the major photoinitiating systems producing reactive radical and ionic species according to the following reaction will be described.
![]()
The possibility of the use of the photoiniting systems in controlled polymer stynthesis will also be discussed. From the practical point of view, these sytems are also attractive in the preparation of block copolymers, because low temperature conditions, usually room temperature, prevents side reactions leading to the formation of homopolymers.
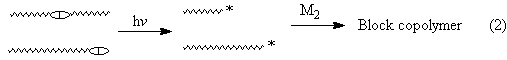
Functionalization of polymers is also achived by photolysis of light sensitive groups that are incorporated into polymers.
![]()
RODERIC P. QUIRK
717 Goodyear Polymer Center, Maurice Morton Institute of Polymer Science, The University of Akron, Akron, Ohio, USA
One of the unique aspects of alkyllithium-initiated, living anionic polymerization is the ability to prepare omega-functionalized polymers by post-polymerization reactions with electrophilic reagents. A particular challenge has been to develop methods that work in hydrocarbon solution at room temperature or higher temperatures, i.e. under conditions in which polydienes with high 1,4 microstructure can be obtained. Although a variety of functionalization reactions have been reported, many of these specific functionalization reactions are either inefficient or have not been adequately characterized. A variety of efficient methods have been developed for preparation of omega-hydroxyl-functionalized polymers including ring-opening reactions of epoxides and oxetanes.
An alternative to these specific functionalization procedures is to find general, quantitative functionalization reactions that are effective for a variety of different functional groups. For example, the reaction of polymeric organolithium compounds with substituted 1,1-diphenylethylenes has been shown to be a useful, general functionalization reaction for a variety of functional groups. The reaction of polymeric organolithium compounds with substituted alkyl chlorides has been investigated as another general functionalization reaction. Although these functionalization reactions are not quantitative in hydrocarbon solution, yields have been improved by optimization of reaction variables such as mode of addition, temperature, addition of Lewis bases and lithium salts and the nature of the chain-end.
A simpler, quantitative functionalization methodology utilizes functionalized alkyllithium initiators. The utility of alkyllithium initiators with protected hydroxyl groups has been investigated for preparation of functionalized polymers (monofunctional, telechelic, heterotelechelic and functionalized, star-branched) in hydrocarbon solution. Amine-functionalized initiators have also been investigated. The scope and limitations of these different functionalization methodologies will be discussed with emphasis on new, efficient procedures.
NIKOS HADJICHRISTIDIS
Dept. of Chemistry, University of Athens, Panepistimiopolis,
Zografou 15771, Athens, Greece
The synthesis and bulk properties of linear block copolymers of styrene and isoprene, having single dimethylamine and sulfobetaine groups at the PS/PI junction point and terminally functionalized PBd stars with one, two or three groups are discussed. Copolymers and star homopolymers were synthesized by anionic polymerization high vacuum techniques. The dimethylamine group at the junction point was introduced after the polymerization of styrene using 1-(4-dimethylaminophenyl)-1-phenylethylene as a capping agent followed by the polymerization of isoprene. The same group was introduced at the chain end by using dimethylaminopropyllithium as initiator. This group was converted to a sulfozwitterrion by reaction with cyclopropane sultone.
The bulk properties of the interfacially modified block copolymers were studied extensively by SAXS, rheology and dielectric spectroscopy. An increase in the order-disorder transition temperature was noticeable for the SZwI copolymers , in accord with theoretical predictions. A new dielectric active process, related to the relaxation of the ionic aggregates at the interface, has been identified by dielectric spectroscopy.
The structure and dynamics of model mono-, di-, and tri-w -functionalized three arm star polybutadiene melts were studied by using X-ray scattering and dynamic rheological measurements. The monofunctional samples behave like multiarm nonionic star-like dendrimers, whereas the difunctional ones resemble a behavior of a transient network consisting of highly branched structures. Finally, the trifunctional stars seem to develop an unusual regular structure of probably intramolecularly aggregated functional groups leading to collapsed star conformations.
AKIRA HIRAO
Department of Polymer Chemistry, Tokyo Institute of Technology, 2-12-1, Ohokayama, Meguro-ku, Tokyo 152-8552, JAPAN
Precise syntheses of various polystyrenes and block copolymers with monosaccharide residues as side chains by means of anionic living polymerization of the following protected monomers are described.1 The resulting polymers all thus
obtained are observed to possess well-controlled structures with respect to molecular weight, molecular weight distribution, and composition of blocks. Stabilities of the living polymer anions derived from these monomers are elucidated by the results of post polymerization and block copolymerization.
Well-defined polystyrenes and polyisoprenes functionalized with a definite number of monosaccharide residues at the chain ends or in the chains are also synthesized2 by living functionalization using their anionic living polymers and the 1,1-diphenylethylene and benzylchloride derivative containing acetal-protected glucofuraose as shown bellow
References
(1) M. Hayashi, S. Loykulnant, A.Hirao, Macromolecules 1998, 31, 2057.
(2) S. Loykulnant, M. Hayashi, A.Hirao, Macromolecules 1998, 31, 9121.
P.J. LUTZ
Institut Charles Sadron, CNRS/ULP 67083 STRASBOURG
Over the years well-defined macromolecules of various architectures could be designed: linear polymers, block copolymers, branched polymers, cyclic polymers, networks with elastic chains of known length…. Their synthesis owes much to anionic polymerization processes whereby the molar mass, the composition in the case of copolymers and/or the structural parameters could be controlled in advance. That polymerization procedure is restricted to a limited number of monomers and has to be conducted under severe experimental conditions.
The main of the present work is to compare the efficiency of various polymerization methods in macromolecular engineering and to discuss advantages and drawbacks of the different methods. For the synthesis of cyclic polymers or of star-shaped polymers ionic polymerization remains the most powerful one, free radical polymerization cannot be applied. One the contrary controlled free radical polymerization has been shown to be almost as efficient as anionic polymerization in designing linear polymers or even copolymers. The present work examines the polymerization of mono or bifunctional macromonomers with respect to the polymerization method. Classical free radical polymerization in homogeneous medium is of limited efficacy for the homopolymerization of monofunctional macromonomers due to the low concentration of polymerizable units. Anionic polymerization is much more efficient. This can be explained by to the long life time of the active sites in the last case. The presence of remaining impurities in the macromonomers limits the domain of applicability of anionic polymerization. The controlled free radical polymerization constitutes an interesting alternative for the homopolymerization of these monofunctional macromonomers. In the case of bifunctional macromonomers anionic polymerization can be applied. Advantage was taken of the combination of a free radical polymerization process with a reaction in heterogeneous medium to design networks and especially hydrogels of controlled elastic chain length. Some biomedical applications of these networks will be discussed.