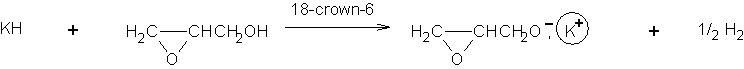
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
ANALYSIS OF GRAFT COPOLYMERS FORMED IN THE PROCESS OF STYRENE DISPERSION POLYMERISATION BY FTIR SPECTROSCOPY
A.YU. MENSHIKOVA, B.M. SHABSELS, E.N. VLASOVA, YU.O. SKURKIS, T.G. EVSEEVA
Institute of Macromolecular Compounds of Russian Academy of Sciences,
Bolshoi 31, 199004 Saint Petersburg, Russia, e-mail: asya hq.macro.ru
Dispersion polymerization of styrene in ethanol solutions of poly-N-vinylpyrrolidon (PVP), acting 4,4¢ -azo-bis-(4-cyanisovaleric acid), is the suitable method to prepare monodisperse carboxylated polystyrene (PS) microspheres, which can be applied as carriers of bioligands or sorbents. The main feature of the process is grafting PVP-chains with polystyrene radicals. As a result, formed PS particles are stabilized due to the electrical charge of their ionized surface carboxyl groups originated from the initiator as well as due to steric repulsion of PVP chains anchored with PS side chains into the PS surface. Properties of PS/PVP graft-copolymers influence particle diameters and final size distribution. To analyze surface layers of PS particles, obtained in conditions of various concentrations of styrene and PVP in the reaction mixture at different solvent contents, FTIR spectroscopy was applied. Previously PS microspheres were thoroughly washed with ethanol or NaOH ethanol solutions to extract PS/PVP graft-copolymers. Then polymer fractions removed from the surface were analyzed by stepwise thin-layer chromatography, which proved the absence of homopolymers. Surface carboxyl groups before and after washing as well as their concentration in washing solutions were determined by conductometric titration.
Analysis of FTIR spectra of the PS/PVP samples was based on characteristic lines of C=O deformation oscillations of PVP lactam groups (1674 cm–1) and of oscillations С-Н of PS benzene rings (700 cm-1). However, in the case of high concentration of PS in the obtained samples, PVP indicating line shifted down to 1656 cm–1, which might be caused by interaction of PVP and PS chains. Mass ratio PVP and PS parts in graft-copolymers estimated from IR spectra was found about (0.8-2):1. The average number and lengths of PS chains grafted to one PVP chain were calculated, considering the titration data in account of one terminal carboxyl group on each PS side chain. The average number of PS chains grafted to one PVP chain varied from 2 to 10, accordingly, the average quantity of monomeric units in each PS side chain changed from 100 to 30 and the distance between grafting sites lied in the range of 100 – 25 monomeric units. Thus, specific control of synthetic conditions allows to obtain PS particles with various morphology of their surface layer. Particularly, carboxylation degree, structure of PS/PVP graft-copolymers and the lenght of hydrophilic PVP tails or loops can be changed.
COMPARATIVE STUDY OF POLYSILOXANE-g-TiNbO5 NANOCOMPOSITES PREPARED BY SEVERAL DIFFERENT ROUTES
A. BEIGBEDERa, S. BRUZAUDa, J. SPĚVÁČEKb, J. BRUSb, Y. GROHENSa
aUniversité de Bretagne-Sud – Laboratoire Polymères et Procédes, Rue Saint Maudé, 56321 Lorient Cedex, France
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, 162 06 Prague 6, Czech Republic
Nanocomposites are hybrid organic-inorganic materials with at least one dimension in the nanometric range. Organic-inorganic hybrid materials have improved mechanical, thermal or barrier properties and fire resistance, for example, compared to pure polymers1. The main drawback of polysiloxanes is their poor mechanical performances and their limited barrier properties. In our original approach we used synthetic mineral oxide HTiNbO5, which is a compound chosen for its perfect lamellar structure and well-defined surface chemistry2.
Three routes were investigated to improve exfoliation of the layered mineral in polysiloxanes:
1) Mechanical shear was applied on polysiloxane filled by untreated HTiNbO5 in order to get a reference of the dispersion state.
2) Anionic polymerization was used to graft polysiloxane onto TiNbO5 layers obtained after an acid-base reaction between HTiNbO5 and (R4N)+;OH-.
3) Different alkoxysilanes were grafted onto TiNbO5 by direct reaction in solution.
The first structural investigations that were carried out on these materials were X-ray diffraction, Differential Scanning Calorimetry and Transmission Electronic Microscopy analysis3. The informations obtained in tension mechanical tests are in good agreement with an improvement of polysiloxanes mechanical reinforcement.
Finally, the effect of grafting, interacting chains or confined chains on the mobility of the polymer is studied. The relation between local mobility that can be probed by CP-MAS solid state 29Si and 13C NMR and the Tg and visco-elastic properties is also tremendous and raises many up to date questions such as the importance of cooperativity in the chain dynamics. Comparison in the T1, T1r and TCH relaxation times will be made according to the method used for the nanocomposite processing. The grafting density, homogeneity or chain length at the surface of the filler will be partially deduced from these investigations.
1 M. Alexandre, P. Dubois, Mater. Sci. Eng., R28, 1 (2000)
2 H. Rebbah, G. Desgardin, B. Raveau, Mater. Res. Bull., 14, 1125 (1979)
3 S. Bruzaud, G. Levesque, Chem. Mater., 14, 2421 (2002)
cancelled
STUDY OF THE HOMOPOLYMERIZATION OF POTASSIUM
GLYCIDOXIDE IN TETRAHYDROFURAN SOLUTION
B. MOREJKO-BUZa,b, A. STOLARZEWICZa,, Z. GROBELNYa; H. FREYc
aInstitute of Physics and Chemistry of Metals, University of Silesia, Katowice,
bCentre of Polymer Chemistry, Polish Academy of Sciences, 41-819 Zabrze, Poland
cInstitute of Organic Chemistry, University of Mainz, 55-128 Mainz, Germany.
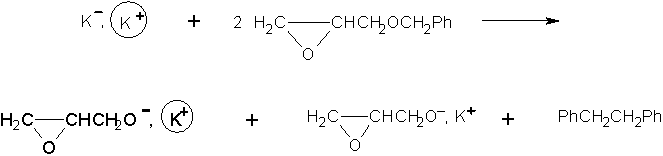
Branched or star-shaped polyethers have traditionally been synthesized with the use of glycidol [1]. For example copolymers of propylene oxide with glycidol have been obtained in this way [2]. However, it was recently reported that branched poly(propylene oxide) and poly(benzyl glycidyl ether) could be prepared in the presence of potassium glycidoxide as the inimer. Two ways of synthesis of potassium glycidoxide were presented below [3]:
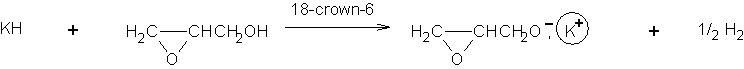

Alkoxide groups were formed which are able to participate in ring opening processes. The initiator system used in the case of 2 was investigated in THF solution. It was found that oligoethers were formed in this process. The molecular weights of the oligomers were around 600 g/mol. NMR spectra confirmed that there were no signals of oxirane ring, suggesting a cyclic structure of its molecules. Electrospray mass spectrometry and MSn experiments were performed to provide an explanation for the cyclic structures formed.
1.Sunder A, Hanselmann R, Frey H, Mülhaupt R. Macromolecules 1999; 32: 4240.
2. Sunder A, Mülhaupt R, Frey H. Macromolecules 2000; 33: 309.
3.Grobelny Z, Stolarzewicz A, Morejko-Buż B, Maercker A. J. Organomet. Chem.,2002; 660, 6.
NMR RELAXATION DISPERSION OF NATURAL RUBBERS UNDER UNIAXIAL DEFORMATION AND SWELLING
S. KARIYO, S. STAPF, B. BLÜMICH
RWTH Aachen, Institut für Technische Chemie und Makromolekulare Chemie, Worringerweg 1, D-52056 Aachen, Germany
The molecular dynamics of polymer melts with molecular weights above the critical value have been described by a power law dependence of the spin-lattice relaxation time on the Larmor frequency which is explained, for instance, by the Doi/Edwards limits of time dependence of mean square displacement of a chain segment. Four different regimes of power laws describing the relaxation dispersion of polymers are distinguished and have been observed experimentally for several types of polymer. In case of natural rubber, the observed relaxation dispersions at low field which can be described as a region of semi-local motion according to those power laws were shown to have the same value of the exponent as observed for the linear polyisoprene with high molecular weights; T1 ~ n g with g » 0.16 at low field and g » 1.2 at high field. These values were left unchanged by increasing the cross-link density.
In this study, the influence of swelling and deformation on the relaxation dispersion of the same natural rubber samples was investigated using field cycling relaxometry. At the same time, the results were also compared to the T1, T2, and double quantum measurements at high field. The relaxation dispersion curves under uniaxial deformation with elongation ratios of l > 1 (stretching) and l < 1 (compression) of natural rubber were obtained. The stretching axes were set to be either parallel or perpendicular to the magnetic field, B0, while the compression axis was only parallel to B0. At high field, the relaxation times remained virtually unchanged while they were reduced at low field. The exponents were found to increase gradually up to the maximum observed value, g » 0.24, under the maximum stretching ratio of this measurement, l » 7. The influence of different degrees of swelling controlled by a mixture of two solvents, on the relaxation dispersion of three natural rubber samples with different cross-link density was observed and compared to equivalent solutions of polyisoprene. At low field, relaxation times were increased with increasing degree of swelling. A transition from a power-law behavior to Rouse dynamics was observed for swollen and dissolved samples. The results are discussed together with high-field relaxation and double quantum data.
INFLUENCE OF COPOLYMERIZATION AND GRAFTING ON THE MOBILITY OF POLYMERS: NMR RELAXOMETRY INVESTIGATIONS
S. STAPF, S. KARIYO, M. WANG, M. BERTMER, B. BLÜMICH
RWTH Aachen, Institut für Technische Chemie und Makromolekulare Chemie, Worringerweg 1, D-52056 Aachen, Germany
The molecular dynamics of polymer melts is described over a wide range of times, or frequencies, by the Doi/Edwards and related theories. Above a critical molecular weight Mc, the time-dependence of the mean-squared displacements of the chain segments is given by characteristic power-law dependences. Because of the coupling between translational and reorientational motion, this affects the frequency dependence of the NMR relaxation time, T1(), which was found to obey similar power-laws.
Reducing the molecular degrees of freedom of the chains, e.g. by introducing cross-links between different segments, was shown before to affect the timescale of motion but leaves the reorientational dynamics virtually unchanged. However, cross-links do not represent perfectly immobilized anchor points. Such fixations can be generated by chemical bonding to a solid object. Copolymerization and grafting have been investigated as two cases of immobilizations by chemical bonding.
First, block copolymers of polybutadiene (PB) and polystyrene (PS) of narrow molecular weight distributions were used which phase-separate into lamellar structures. The PB mobility is shown to be reduced with decreasing chain lengths for diblock copolymers PS-PB, the effect being even stronger for triblocks PS-PB-PS. The power-law dependence which was found for pure bulk PB, and thus the statistics of motion, was observed to be changed in the block copolymers, in particular the range of the intermediate region of tube-affected motion was reduced in width. Second, Polydimethylsiloxane (PDMS) was grafted onto SiO surfaces, which generated short loops of typically less than 10 monomer units between grafting points. Increasing grafting density led to the complete suppression of the above mentioned intermediate frequency regime, while the high-frequency behaviour (> 107 Hz) remained mostly unaffected. The temperature dependence of the relaxation dispersion of phase-separated and grafted polymers is presented and is compared with longitudinal and transverse relaxation time measurements at high magnetic fields.
SPECTROSCOPIC STUDY OF THE STRUCTURE OF CROSSLINKED POLY(AMIDE-IMIDE)S
P.SYSEL, V. ŠINDELÁŘ, Š. NEJEDLÁ, R. HOBZOVÁ
Department of Polymers, Institute of Chemical Technology, Technická 5,
166 28 Prague 6, Czech Republic, Petr.Sysel vscht.cz
Aromatic polyimides have found many applications in (micro)electronics, aviation industry and membrane technologies due to their excellent thermal, mechanical and dielectric properties. For some of these applications the properties of polyimides can be improved by their modification and crosslinking [1].
This work deals with the characterization of crosslinked poly(amide-imide)s [2]. Polyamic acids based on aromatic dianhydrides and aromatic diamines were used as the starting polyimide components. The 4-methyl-1,3-phenylene diisocyanate terminated poly(ethylene adipate)s and poly(ethylene glycol)s with a number average molecular weight ranging from 1000 to 3500 g/mol were selected as the modifying components. The reactive isocyanate groups of oligomers were blocked by the reaction with phenol. Crosslinked polymeric materials are not processable and thus the solution of polyimide and modifying components was cast onto the substrate as a thin layer before the thermal exposition. Materials with the theoretical content of modifying component ranging from 20 to 80 wt.% were prepared. The final films were analyzed using IR spectroscopy.
The termination of oligomers with 4-methyl-1,3-phenylene diisocyanate and the reaction of isocyanate group of oligomers with the groups of polyimide components were studied with the utilization of low-molecular substances. The products of model reactions were analyzed using NMR and IR spectroscopy. It was found that the crosslinking is arranged by the reaction of isocyanate groups with the hydrogen of carbonyl and amide groups of polyamic acids.
This work was supported by the Grant Agency of the Czech Republic (Grant 104/03/0338) and by the project CEZ:MSM 223100002.
1 C.E. Sroog: Prog. Polym. Sci 16, 561 (1991).
2 V. Šindelář, P. Sysel, V. Hynek, K. Friess, M. Šípek, N. Castaneda: Collect. Czech.Chem. Commun. 66, 533 (2001).
FTIR SPECTROSCOPY OF PARTIALLY ORDERED CONDUCTING POLYANILINE FILMS
M. TRCHOVÁa, I. SAPURINAb, J. STEJSKALa
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, 162 06 Prague 6, Czech Republic
bInstitute of Macromolecular Compounds, Russian Academy of Sciences, St. Petersburg 199 004, Russia
A thin surface layer of a conducting polymer, viz. polyaniline, deposited on varuious materials by polymerization is often needed for practical applications. The concept of brush-like ordering of macromolecules chains in produced films has been proposed in [1]. The influence of the polymerization conditions on self-assembling of polyaniline chains in the films via hydrogen bonding has been studied by FTIR spectroscopy.
Polyaniline films were produced by the oxidation of aniline hydrochloride (0.20 M) with ammonium peroxydisulfate (0.25 M) in dilute hydrochloric, sulfuric and phosphoric acids. The films were obtained in situ on various surfaces immersed in the reaction mixture. The various spectroscopic techniques used included transmission measurements of films deposited on silicon wafers, attenuated total reflection applied to films grown on polyethylene supports, and reflection from films prepared on gold mirrors.
Five, so-called ”H-peaks” located at 3220, 3140, 3050, 2920, and 2830 cm–1 correspond to the hydrogen bonding between regularly aligned PANI chains. They are independent of the support and mode of measurement. These absorption peaks remain unchanged during the heating of films to 120oC. Thus it seems that the absorption bands in the 3300–2800 cm–1 region reflect the organization of PANI chains within the film by hydrogen bonding involving NH and NH+ groups [2]. The shapes of the spectra prepared in the presence of different acids have been compared. Chloride, sulfate and phosphate counterions influence the self-assembling of polyaniline chains.
Acknowledgement. The research was supported by the Grant Agency of the Czech Republic (202/02/0698) and the Grant Agency of the Academy of Sciences of the Czech Republic (A 4050313).
[1] Sapurina I., Osadchev A.Yu., Volchek B.Z., Trchová M., Riede A., Stejskal J.: Synth. Met. 129 (2002) 29.
[2] Trchová M., Sapurina I., Prokeš J., Stejskal J.: Synth. Met. 135–136 (2003) 305.
MULTIQUANTUM AND SPIN DIFFUSION EXPERIMENTS ON GRAFTED PDMS
M. WANG, M. BERTMER, D. E. DEMCO, B. BLÜMICH
Institute for Technical Chemistry and Macromolecular Chemistry, RWTH Aachen, Germany
Grafted polymers are a relatively new class of materials that are of increasing importance i. e. in surface coating and have therefore widespread applications. We used solid state nuclear magnetic resonance to study the effect of grafting on the polymer mobility and segmental orientation. Our system consists of short chains of poly(dimethylsiloxane) (PDMS) with different number of monomer units chemically attached to hydrophilic silica. The methods used exploit of homonuclear as well as heteronuclear residual dipolar couplings. Different pulse sequences were applied such as proton double- and triple-quantum excitation for the homonuclear, spin-echo double resonance and heteronuclear double quantum excitation for the 13C{1H} heteronuclear case. Together with numerical simulations we deduce the heterogeneity of the order parameter along the chain, which is related to the overall chain length and compare the different measurement techniques.
Two components are present in the proton spectra – a mobile one which is assigned to the free chain and a more rigid one assigned to an interface region at the grafting point - we additionally measured double-quantum coherences as a function of temperature to deduce the temperature dependency of the two components. Spin diffusion techniques are used to measure the macromolecular fragment sizes of the grafted polymers and to correlate the results with other measurements of the heterogeneity of residual dipolar couplings. With all these experiments we get an overall picture of the mobility and segmental orientation of PDMS on fumed silica.
POLYMER ELECTROLYTE PEO/LiCF3SO3 AND OTHER INTERCALATE PEO COMPLEXES STUDIED BY SOLID STATE 1H AND 13C NMR SPECTROSCOPY AND DFT CALCULATIONS
J. SPĚVÁČEK, J. BRUS, J. DYBAL
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, 162 06 Prague 6, Czech Republic; spevacek imc.cas.cz
It is well known that poly(ethylene oxide) (PEO) and alkali metal salts can form molecular complexes that exhibit high ionic conductivity and therefore are called solid polymer electrolytes. In the present work we investigated the PEO/LiCF3SO3 complex. Using methods that make it possible to separate 1H and 13C NMR spectra of amorphous and crystalline phases of this complex, interactions in both phases were characterized by 1H and 13C chemical shifts of PEO. A lower shielding of PEO protons and higher shielding of PEO carbons was found for the PEO/LiCF3SO3 complex compared with the neat PEO. Both 1H and 13C chemical shifts were virtually the same for PEO in amorphous and crystalline phases, showing the same interaction of PEO with LiCF3SO3 in both phases, and indicating essentially the same local structure in both cases. These results are corroborated by quantum-chemical DFT calculations on model complex of diglyme CH3O(CH2CH2O)2CH3 and LiCF3SO3. In accord with experimental results, these calculations have shown a lower shielding of diglyme protons and a higher shielding of diglyme carbons in the model complex. The structure of the model complex agrees well with the crystal structure of the PEO/LiCF3SO3 complex as determined by X-ray diffraction. Measurement of the dynamics of the Lee-Goldburg cross-polarization 1H® 13C was used to determine the distance between the LiCF3SO3 carbon and its nearest PEO protons in the complex.
Intercalate crystalline complexes of PEO and hydroxybenzenes, stabilized by hydrogen bonds, were also studied for comparison. For PEO/4-nitrophenol and PEO/resorcinol complexes with strongest hydrogen bonds, changes of 1H and 13C chemical shifts (compared with neat PEO) in opposite directions were found, similarly to those for the PEO/LiCF3SO3 complex. 2D 1H CRAMPS exchange spectra as a function of the mixing time were used to characterize proton distances in PEO/hydroxybenzene complexes.
Acknowledgment: Support of the Grant Agency of the Academy of Sciences of the Czech Republic (grant No. IAA4050209) is gratefully acknowledged. The authors thank Prof. M. Dosiere and Dr. J.-F. Moulin (Université Mons-Hainaut, Belgium) who kindly provided the samples.
1H NMR study of thermotropic phase transitions in D2O solutions of poly(N-isopropylmethacrylamide)/ poly(vinyl methyl ether) mixtures
L. Starovoytovaa, J. Spěváčeka, b , L. Hanykováa, M. Ilavskýa, b
a Charles University, Faculty of Mathematics and Physics, V Holešovičkách 2, 180 00 Prague 8, Czech Republic
b Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, 162 06 Prague 6, Czech Republic
Poly(N-isopropylmethacrylamide) (PIPMAm) and poly(vinyl methyl ether) (PVME) in aqueous solutions undergo the phase transition at a lower critical solution temperature (LCST). While below LCST polymer chains are flexible and exhibit coil structure, heating of solutions above the LCST results in the phase separation (chains exhibit more compact globular structure) due to a change in balance between various polar and non-polar interactions. 1H NMR methods were applied to characterize polymer-polymer and polymer-solvent interactions during the temperature-induced phase transition in mixtures of PVME (LCST » 308 K) and PIPMAm (LCST » 315 K) in D2O. The solutions were investigated in the range of polymer concentrations c = 0.1 – 10 wt.-% and with various compositions (molar ratios) of mixtures.
Above the LCST transition, a reduction in the absolute integrated intensities was observed for polymer bands in high-resolution 1H NMR spectra due to a pronounced decrease of mobility of phase-separated polymer units. Two phase transitions were detected in all investigated PVME/PIPMAm/D2O mixtures corresponding to different LCSTs of individual components. While the temperature region of the first transition of PVME component was almost independent on the polymer concentration and the composition of the mixture, the second transition of PIPMAm component was markedly influenced by the both parameters. Measurements of the spin-spin relaxation of residual water (HDO) revealed that above the phase transition, a part of water molecules is bound to globular structures.
Acknowledgment: This work was supported by the Grant Agency of the Charles University (grant No. 164/2001B) and the Grant Agency of the Academy of Sciences of the Czech Republic (project No. IAA4050209).
CHARACTERIZATION OF POLY(N-ISOPROPYLACRYLAMIDE) IN VARIOUS SOLVENTS STUDIED BY STATIC AND DYNAMIC LIGHT SCATTERING
H. SATO1, Y. KATSUMOTO1, K. KUBOTA2, Y. OZAKI1
1School of Science and Technology, Kwansei-Gakuin University, Sanda, Hyogo 669-1337, Japan
2 Faculty of Engineering, Gunma University, Kiryu, 376-8515, Japan
Poly (N-isopropylacrylamide) (PNiPA) is well known to show a volume phase transition with temperature change. The structure and property of PNiPA in solutions are sensitive to environment, such as temperature, pH and solvent composition. PNiPA chain has a soluble-precipitation-soluble property in the mixture of water and methanol. In addition, PNiPA chain shows solubility in various solutions. The purpose of this study is to explore the interaction between PNiPA molecule and solvent in various solutions. Furthermore, we try to compare the results from the light scattering study with those of our infrared study.
Various molecular parameters characterizing the solution properties of PNiPA in several solvents (THF, acetone, 1-propanol, chloroform, methanol and methanol / water) were investigated by using static and dynamic light scattering technique. The weight averaged molecular weight, Mw, second virial coefficients, A2, and radius of gyration, Rg, were estimated from Berry’s plot (static light scattering) of PNiPA in methanol at 25oC. PNiPA molecules behave as a highly hydrated flexible coil in water at apart from the transition temperature (ca. 32oC). On the other hand, the polymer chain behaves as fully interpenetration in methanol. The value of A2 of PNiPA in the mixture of water and methanol changes dramatically with a small variation in the solvent mixture ratio. The polymer molecules exhibit a strong selective solvation effect in the mixed solvent. It is shown that the water molecules exist around the PNiPA molecules selectively in the mixed solvent. In methanol and THF, Rg and hydrodynamic radius, Rh, are smaller than those in water, and A2 is positive but smaller than that in water, suggesting that water at 25oC should be a better solvent than methanol and THF from the viewpoint of A2. Interpenetration function Ψ of PNiPA is different for each solvent. It suggests that PNiPA has a difference local interaction with each solvent. Furthermore, the figure of the interpenetration function Ψ versus expansion coefficient separates in to two parts approximately.
NMR FRINGE FIELD DIFFUSOMETRY ON POLYMER MELTS OF HIGH MOLECULAR WEIGHTS IN NANOPOROUS METHACRYLATE MATRICES
E. FISCHERA, R. KIMMICHA, U. BEGINNB
a Sektion Kernresonanzspektroskopie, Universität Ulm, D-89069 Ulm, Germany
b ITMC/Tex MC, RWTH Aachen, Worringerweg 1, D-52056 Aachen, Germany
The dynamics of polymer chains confined to artificial tubes formed by the pores of a nanoporous material should display all the features predicted by the reptation model [1]. For a polymer melt inside a porous medium, only a motion along a curvilinear fixed path is possible leading to a behaviour similar to region III of the tube/ reptation picture.
In order to study the dynamic interaction between a given chain and the restricting pore wall, the investigated polymer should be of high molecular weight, i.e. chain-end effects can safely be neglected. The porous matrix, on the other hand, should consist of a material with narrow elongated pores of preferably cylindrical shape.
A material that fulfils these requirements is generated by the polymerisation of a monomer solution of hydroxymethylacrylate containing poly(ethylene oxide) [2]. Cooling down a homogenous solution of poly(ethylene oxide) in hydroxy-methylacrylate monomers spinodal phase separation takes place and creates phase boundaries between the polymer and the monomer molecules. The poly (ethylene oxide) in the polymer-rich phase vitrifies and can be preserved by subsequent polymerisation and cross-linking of the monomers to build the later matrix of poly(hydroxymethacrylate). Elevating the temperature above the melting temperature of the polymer leads to the situation in focus: a polymer melt moving in a nanoporous solid matrix.
Fringe field diffusometry has been performed on poly(ethylene oxide) melts in porous material prepared as previously described [3]. The influence on molecular dynamics by the presence of the solid tube wall favourably compares with predictions of the tube/reptation model, whereas bulk melt data are not compatible in detail with that theory.
[1] M.Doi, S.F. Edwards, Theory of Polymer Dynamics, Clarendon Press, Oxford, 1986.
[2] E. Fischer, R. Kimmich, U. Beginn, M. Möller, Phys. Rev. E. 59, 4079–4084 (1999).
PARTIAL ORDERING OF H2O AND POLYMER MOLECULES IN THERMOTROPIC MATERIALS
D. KURKOVÁ, J. KŘÍŽ, P. SCHMIDT, J. DYBAL
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovský sq. 2, CZ-162 06 Praha 6, Czech Republic
Three types of polymers with thermotropic properties were studied by 1H, 13C NMR spectroscopy, T1, T2 NMR relaxometry and PGSE self-diffusivity. Polymers under investigation were poly(GVGVP), poly(AVGVP), and [(PGVGV)2-(PGEGV)-(PGVGV)2]9 , where A: alanine, V: valine, G: glycine, P: proline, E: glutamic acid. All of them were in H2O or D2O solutions. Measurements were performed in the temperature interval from 10 to 50 ºC and at 5% and 0.5% w concentrations. The transition temperature was observed in the region of 27 – 33 ºC.
With respect to results of our experiments and also of literary data it was found that the effect of ordering of polymers and H2O molecules play a role in thermotropic properties of these materials.
The poly(AVGVP) with more hydrophobic side chains turns into a relatively rigid molecular form in a narrow temperature interval. In contrast the physical transformation of poly(GVGVP) with less hydrophobic side chains proceeds over wider temperature interval. Here the most pronounced intensity changes, corresponding to the increased rigidity of the polymer, are observed for protons directly attached to the polymer backbone, whereas the more distant protons reach the immobilized state less easily. 13C T1 relaxation data and its temperature changes also support the idea of increased rigidity of the polymer at higher temperatures.
The hydrodynamic ratio of the polymer molecules calculated from the self-diffusion coefficients at higher concentration (5%w) of the polymer decreases with temperature, at lower concentration (0.5%) it remains constant. This is in agreement with 1H intensity changes. We can conclude that at higher concentration polymer coiling due to intermolecular interactions takes place; at lower concentration polymer is more extended and the intramolecular interactions are prevalent.
The temperature course of HOD T1 and T2 relaxation times, after its analysis indicate also ordering of HOD molecules in these systems.
Acknowledgement: The authors wish to thank to the Grant Agency of the Academy of Sciences of Czech Republic (Projects No.: AVOZ4050913, KSK4050111, A4050208 ).
Abstract not supplied
THE VARIATION OF THE HOMOPOLYPEPTIDE STRUCTURE CAUSED BY INTERACTION WITH THE PYRROLE DERIVATIVES. VCD STUDY
M. URBANOVÁa, L. PALIVECb, V. KRÁLb, K. VOLKAb
aDepartment of Physics and Measurements, bDepartment of Analytical Chemistry, Institute of Chemical Technology, Technická 5, CZ-166 28 Praha 6, Czech Republic
It is well known that homopolypeptides, as poly-L-lysine, poly-L-glutamic acid, and poly-g -benzyl L-glutamate, reveal different structures depending on pH, temperature, history of preparation, and addition of salts. Polypeptides, which contain functional groups that are subject to protonation and deprotonation, can also serve as partners in non-covalent interactions with appropriate molecules with anionic and cationic groups, respectively [1]. Such interactions can influence the structure of matrices as well. The goal of this contribution is to study the effect of the both factors, physicochemical conditions and interactions, on local and global molecular organizations of the polypeptide matrices.
 In this study we
employed vibrational circular dichroism (VCD) spectroscopy that
is very sensitive to structure of chiral molecules. The subtle
variation in structure caused by interactions can be followed
through the changes of the shape of the VCD spectra.
In this study we
employed vibrational circular dichroism (VCD) spectroscopy that
is very sensitive to structure of chiral molecules. The subtle
variation in structure caused by interactions can be followed
through the changes of the shape of the VCD spectra.
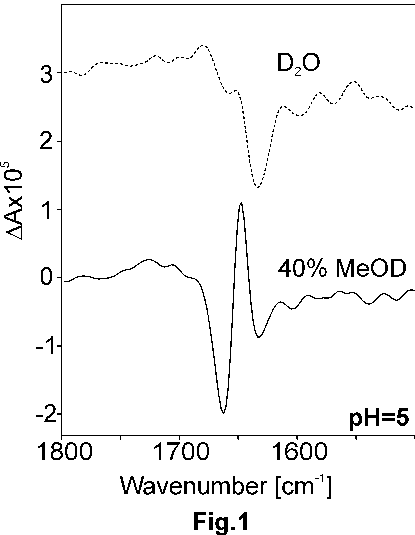
In Fig. 1, as an example of presented study, VCD spectra of poly-L-glutamic acid with the derivative meso-tetra(4-N,N,N-benzyl-trimethyl-ammonium)porphine tetrabromide in D2O solutions, pH = 5, and in mixed D2O/MeOD solution are shown. The result is interpreted as the conformational change of the matrix from the random-coil to a -helical structure. The presence of the porphyrin derivative is crucial as in the absence of porphyrin, the precipitation occurs.
Acknowledgments: Grant CEZ: MSM 223400008 from the Ministry of Education, Youth and Sports of the Czech Republic supported the work.
[1] Urbanová M., Setnička V., Král V., Volka K., Biopolymers 2001, 60, 307-316.
COMPLEXATION SELF-ASSEMBLY OF BIARYL DERIVATES: COMPUTATIONAL AND EXPERIMENTAL VCD STUDY
T. KOTRBAa, V. SETNIČKAb, M. URBANOVÁa, K. VOLKAb, S. ZÁLIŠc,
aDepartment of Physics and Measurements, bDepartment of Analytical Chemistry, Institute od of Chemical Technology, Technicka Technická 5, CZ-166 28 Praha 6, Czech Republic
cJ. Heyrovský Institute of Physical Chemistry, Academy of Sciences of the Czech Republic, Dolejškova 3, 182 23 Prague Praha 8, Czech Republic
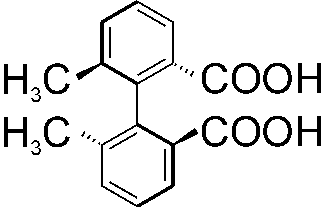
The biaryl derivatives form supramolecular self-assembly
probablyoften composed from the tetramers of bialryls. During
this process, the conformation of the consisting units can
be changed. Because of the high sensitivity of vibrational
circular dichroism (VCD) to such subtle changes, VCD spectroscopy
was employed in the conformational study of isolated units in the
DMSO and alkalic aqueous solutions, and self-assembly in DMSO, in
the CDCl3 solutions., and in alkalic aqueous
solutions. A week VCD signal Absorption and VCD spectra of (S)-)-2,2’-dimethylbiphenyl-6,6’-dicarbo-xylic
acid in carbonyl region is observed in DMSO solutions, where the
self-assembly is prevented, however strong couplet is observed in
CDCl3 solution.
The interpretation of these spectral observations was made by density functional calculations including the solvent effects. The calculations were done for several aggregates of (S)-2,2’-dimethylbiphenyl-6,6’-dicarboxylic acid (monomers, dimmers, trimers and tetramers) in order to explain the possible intermolecular interactions under different conditions. The simulated VCD spectrum of 2,2’-dimethylbiphenyl-6,6’-dicarboxylic acid and its dimer is shown in attached Figure. This The attached Ffigure of simulated VCD spectrum of (S)-2,2’-dimethylbiphenyl-6,6’-dicarboxylic acid and its dimer illustrates the possible influence of the aggregation on the spectral characteristics.
Acknowledgments: Grant CEZ: MSM 223400008 from the Ministry of Education, Youth and Sports of the Czech Republic supported the work.
WATER-POLYMER INTERACTIONS IN POLY(PENTAPEPTIDE)S STUDIED BY NMR, VIBRATIONAL SPECTROSCOPY AND AB INITIO CALCULATIONS
J. DYBALa, P. SCHMIDTa, J. KŘÍŽa, D. KURKOVÁa, J.C. Rodríguez-Cabellob, M. Alonsoc
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovský Sq. 2, 162 06 Praha 6, Czech Republic
bDepartment of Condensed Matter, E.T.S.I.I., University of Valladolid, 47011 Valladolid, Spain
cDepartment of Analytical Chemistry, E.U.P., University of Valladolid, 47014 Valladolid, Spain
Hydration and water-polymer interactions are important factors in the stability of the folded structure of water-soluble polypeptides and proteins. The role of the hydration water in the inverse temperature transition of elastin-derived biopolymers represented by poly(Gly-Val-Gly-Val-Pro) and poly(Ala-Val-Gly-Val-Pro) have been investigated by NMR, ATR-FTIR, Raman spectroscopy and ab initio quantum-chemical calculations. Temperature and concentration dependences of the infrared and Raman spectra have shown that the studied polymers exhibit vibrations sensitive to both changes in the conformational structure of the main-chain and local changes in the environment of polymer chains caused by water-polymer interactions (C-H stretching). The observed frequency shifts of the C-H stretching vibrations are caused by blue-shifting hydrogen bonding.
Temperature and concentration dependences of the Raman and FTIR spectra and the 13C and 1H NMR chemical shifts measured for water solutions of polymers and of low-molecular-weight models have been correlated with ab initio calculations. Vibrational frequencies and intensities as well as 13C and 1H NMR chemical shieldings were calculated at the density functional theory (B3LYP) and second order Møller Plesset (MP2) levels using the 6-31G(d) and 6-311+G(2d,p) basis sets for model structures of polypentapeptides surrounded by a growing number of water molecules, from one water molecule to an ordered clathrate-like water structure. The results will be discussed in relation to the order and dynamics of the hydration water surrounding non-polar groups.
Support of this work by the Grant Agency of the Academy of Sciences of the Czech Republic (Grant No. IAA4050208) is gratefully acknowledged.
STRUCTURAL PECULIARITIES OF ORGANO-INORGANIC NANOHYBRIDS: VIBRATIONAL SPECTROSCOPIC INVESTIGATION
D. OSTROVSKIIa, V. DE ZEA BERMUDEZb, M.C. Gonçalvesb, P. JACOBSSONa , L.D. CARLOSc
aDepartment of Applied Physics, Chalmers University of Technology, SE-41296 Göteborg, Sweden
bDepartamento de Química, Universidade de Trás-os-Montes e Alto Douro, Quinta de Prados, Apartado 1013, 5001-911 Vila Real Codex, Portugal
cDepartamento de Física, Universidade de Aveiro, 3810-183 Aveiro, Portugal
During recent years a new class of nanoscale organic/inorganic frameworks has attracted increasing interest due to wide range of possible technological applications. Such complex systems, basically obtained by the sol-gel technique, include flexible polymer chains grafted at one or both sides to the inorganic backbone via specific cross-links. After doping with an appropriate salt, these materials may demonstrate prominent luminescent or ion conducting properties and have, in addition, higher mechanical stability provided by the inorganic counterpart.
In our previous works we investigated a family of nanohybrid xerogels in which poly(oxyethylene), POE, chains are connected to the siliceous backbone (SiO) through urea (-NHC(=O)NH-) or urethane (-NHC(=O)) bridges. Upon varying different parameters, e.g. the length of polymer chains, the cross-link type and the concentration of the added salt, it is possible to tune the resulting features of the system. It appears, however, that useful properties of the material come to a large extent from the way the host matrix coordinates incorporated cations and anions. This coordinating property, in turn, is determined by the accessibility of the active sites of the matrix (i.e. C=O, NH, POE oxygens) for guest ions. Therefore the knowledge of structural peculiarities of the xerogel framework and mutual coordination between its organic and inorganic parts is a key issue for the development of practical materials.
In this work we apply vibrational spectroscopic techniques (both infrared and Raman) to investigate micro- and macroscopic structure of several systems synthesized under different conditions. The main aim is to elucidate the influence of structural parameters on the coordinating ability of the matrix as well as the system’s structural stability toward increasing addition of guest salt.
MONITORING STRAIN-INDUCED STRUCTURAL CHANGES IN STYRENe COPOLYMERS BY ELECTRON MICROSCOPY AND FTIR SPECTROSCOPY
a R. ADHIKARI, a T. AN HUY, b P. SIMON, a R. GODEHARDT, a W. LEBEK, a G.H. MICHLER
a Institute of Materials Science, Dept. of Engineering Science, University of Halle-Wittenberg, Geusaer Strasse, Geb. 131, D - 06217 Merseburg, Germany
b Max-Planck-Institute for Chemical Physics of Solids, Nöthnitzer Str. 40, D - 01187 Dresden, Germany
Block copolymers and polymer blends have attracted a lot of commercial and academic interest because of their interesting mechanical properties. Precise understanding of morphology formation and structural changes due to external loading in these polymers is essential to adjust their mechanical behaviour.
Electron microscopy is a very important tool in the identification and characterisation of structure and morphology of polymers. In addition to the widely used staining technique using heavy metals, the structural details of heterogeneous polymers may be imaged by strong defocus or electron holography. Recently, scanning force microscopy and electron holography have been successfully used characterise structural details in polymer blends and block copolymers (Simon et al., Macromolecules in Press). The aim of this work is to study comparatively the morphology and strain induced structural changes in individual phases of selected styrenic polymers (high impact polystyrene and block copolymers) by means of scanning force microscopy (SFM), electron holography and Fourier-transform infrared (FTIR) spectroscopy.
INTERNAL REFLECTION SPECTROSCOPY OF GELATIN INTERFACIAL LAYERS
B. TARASEVICH, G. YAMPOLSKAYA, V. IZMAILOVA
Chem. Department, Moscow State University, Moscow, 119899 Russia
Interfacial layers (IL) of biopolymers are of strong interest for different areas of the human activity. In particular these layers play the determing role in the emulsion stabilization. Formation of IL of biopolymers is rather complicated phenomenon, involving transport of macromolecules to the liquid- liquid interface, fixation of them at the surface, orientation according to the hydrophobic-hydrophilic balance, phase separation within thin layer (due to a high biopolymer concentration), and finally formation of interface structures. Relative rates of these processes, thermodynamics and entropy-related contributions depend on both the nature of immiscible liquids and biopolymer.
Despite of numerous studies of IL, we are up to present far from the complete description of the process of the formation and states of IL. MATR (multiple attenuated total reflection) in IR range is powerful instrument for investigation of transferred layers of biopolymers. The systems consisting of aqueous gelatin solution – nonpolar phase (such as CCl4, C6H6, alkanes C6-C12 or air) in which IL are formed were considered. To obtain IR spectra of IL, we used the set up analogous to the Langmuir – Blodgett technique. Ge or Si optic element MATR was submerged into aqueous phase. After formation of IL the element MATR was drew out slowly (1 cm/min) through the interface, simultaneously special barriers push ahead the IL onto the surface of element MATR. Following results of the experimental study are presented:
1) The absorption band of Amide I (1640 cm-1) in IR spectra is the superposition of individual bands corresponding to different conformational states of polypeptide chain: helices, folded structures, random coils. Experimental contour of Amid I was expanded into constituents by computer-aided calculations. It was found that the conformational state of polypeptide chains within adsorption layers depend on pH of aqueous phase. Maximum of macromolecular regularity in IL was observed, when aqueous phase has pH = 4,9 (isoelectric point of gelatin). Under these conditions IL reveal similar stray rheological parameters and apparent thickness. 2) For determing apparent thickness of transferred IL the absorption coefficients of analytical bands were determined by using of special calibration procedure. Apparent thickness of IL depend on time of the IL formation, gelatin concentration and pH of aqueous phase. The length of hydrocarbon chain used as nonpolar phase was found affect the IL thickness. 4) The initial stage of the IL formation is described by the fractal model.
Adsorption of macromolecules (MM) differs from that of low-molecular surface active substances by the fact that MM may be in numerous conformations (both in solution and IL) and that MMs may be immobilized on the surface by several points.
Acknowledgments. This work was supported by Russian Foundation for Basic Research.
TIME-RESOLVED STRUCTURAL CHANGES OF POLY(e -CAPROLACTAM) DURING STRETCHING INVESTIGATED BY ULTRARAPID FTIR SPECTROSCOPY
P. SCHMIDTa* , J. DYBALa, CH. PELLERINb, P. R. GRIFFITHSc
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovský Sq. 2, 162 06 Prague, Czech Republic
bCentre de recherche en sciences et ingénierie des macromolécules, Département de chimie, Université Laval, Québec, Canada, G1K7P4
cDepartment of Chemistry, University of Idaho, Moscow, ID, 83844-2343, U.S.A.
Fast stretching of poly(e -caprolactam) (Nylon 6) was investigated by ultra-rapid FTIR spectroscopy in the time scale of 1.8 s with the time resolution of 10 ms. It was found that in the course of stretching the orientation and conformation changes exhibit an indication of oscillatory behaviour. The content of conformation of the planar chains and the orientation function first increases as a result of increasing stress. After formation of a neck causing the decrease of the tension, both the content of planar conformation and the orientation function decrease. With continuing stretching they increase again and show a hint of another period. After the end of stretching, the formed structures are stabilised. The observed instability of the planar form of poly(e -caprolactam) chains was explained by the existence of a partially-ordered pre-crystalline state, forming a basis for the consecutive formation of stable monoclinic a -crystalline form.
SELF-ORGANIZATION, STRUCTURE, SURFACE MORPHOLOGY AND DYNAMIC PROPERTIES OF SILICA/EPOXY FILMS AS SEEN BY SOLID-STATE NMR, AFM AND SAXS
J. BRUS, M. ŠPÍRKOVÁ, D. HLAVATÁ
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, 162 06 Prague 6, Czech Republic
e-mail: brus imc.cas.cz
Self-organization, structure, surface morphology and segmental dynamic properties of hybrid organic–inorganic (O-I) coating films and free–standing films as determined by various techniques of solid-state NMR, Atomic Force Microscopy (AFM) and Small Angle X-ray Scattering (SAXS) are reported in this contribution. Hybrid O-I epoxide-based products were prepared from functionalized organosilica precursors (i.e. [3-(glycidyloxy)propyl] trimethoxysilane (GTMS) and diethoxy-[3-(glycidyloxy)propyl] methylsilane (GMDES)), oligomeric di- and triamines and in some cases colloidal silica particles so interlinked organic-inorganic networks can be formed. During the early stages of formation of O-I films (hydrolysis and polycondensation of siloxanes) no cleavage oxirane rings was observed. In addition presence of colloidal silica accelerates hydrolysis and condensation reactions. Final distribution of siloxane structure units and their condensation degree strongly depends on the type of organosilica precursor. Differences in self-organization as well as in homogeneity of hybrid I-O networks depending on their composition were detected by solid-state NMR techniques (2D CRAMPS, 2D 1H-13C HETCOR) and SAXS. GTMS containing network is nanoheterogenous internally organized system with long period ca. 2-3 nm. Interaction between organic phase and colloidal silica particles was probed by 2D 1H-29Si HETCOR and 2D 1H-29Si WISE. Segmental dynamics of various systems was determined and evaluated among others according to the series of NMR relaxation measurements (T1(13C) and T1(13C)). Surface morphology and other surface characteristics were determined by AFM.
SOLUBILIZATION IN NANOPARTICLES BASED ON BLOCK COPOLYMER MICELLES: AN NMR AND SANS STUDY
J. PLEŠTILa, J. KŘÍŽa,
P, KADLECa, H. POSPÍŠILa, L.ALMÁSYb,
A.B. KUKLINc
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, CZ-162 06 Praha 6, Czech Republic
bResearch Institute for Solid State Physics and
Optics, POB 49, H-1525 Budapest, Hungary
cFrank Laboratory of Neutron Physics, Joint Institute
for Nuclear Physics, 141980 Dubna, Russia
Most envisaged applications of block copolymer micelles are
based on loading sparingly soluble compounds into micellar cores
(solubilization). A possibility of controlling characteristics of
this process is of great importance for applications. This
contribution illustrates that nanoparticles prepared by
polymerization of methyl methacrylate (MMA) in micellar solution
of polystyrene-block-poly(methacrylic acid) (PS-b-PMA)
rank among promising candidates for the controlled uptake/release
processes. 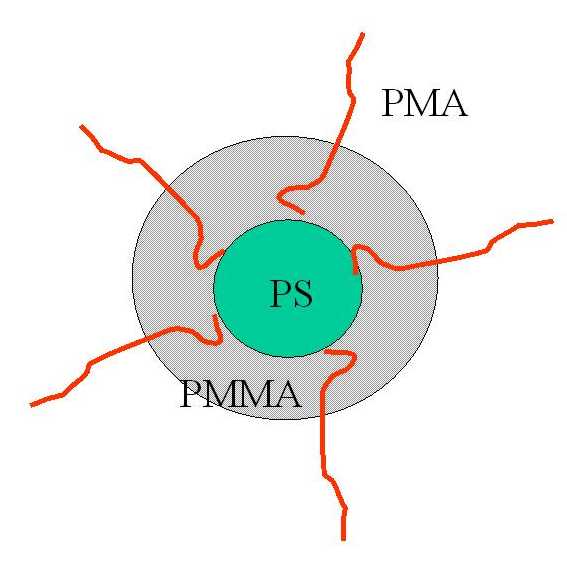
For a series of samples differing in the thickness of MMA
layer (see Figure) we studied solubilization of benzene and
chloroform. Using the time-resolved NMR and SANS experiments
various equilibrium and kinetic characteristics of the process
(solubilization rate and capacity, partition coefficients) were
determined. NMR technique provided information on the mobility of
both the polymer components and solubilizate and on its variation
during solubilization. It was found that transport properties of
the PMMA layer differ from those of micellar cores formed by PS
or PMMA.. This was confirmed by comparison of the time
dependences of the nanoparticle core parameters (volume, radius,
scattering invariant) for normal (H ) and deuterated (D) benzene.
The results demonstrated that the studied type of nanoparticles
could serve as a host for sparingly soluble compounds and their
structural parameters can be finely tuned to optimize the
solubilization properties.
Support of the Grant Agency of the Czech Republic (grant No. 203/03/ 0600) and Grant Agency of the Academy of Sciences of the Czech Republic (grant No. AVOZ4050913) are gratefully acknowledged.