
Enrico Drioli, Enrica Fontananova
Institute on Membrane Technology (ITM-CNR), c/o University of Calabria, Via P. Bucci, 17/C, 87030, Rende (CS), Italy
Department of Chemical Engineering and Materials, University of Calabria, Via P. Bucci, 87030, Rende (CS), Italy.
Tel.: +39 0984 492039; Fax: +39 0984402103; E-mail: e.drioli/itm.cnr.it
A membrane is an interphase that restricts the passage of different components in a specific mode and over a wide range of particle sizes and molecular weights, from ions to macromolecules [1].
Synthetic membranes may be manufactured using organic or inorganic materials; they may be homogeneous or heterogeneous, symmetrical or asymmetrical, porous or dense, electrically neutral or charged; they may exhibit isotropic or anisotropic properties.
The efficiency of a membrane basically is determined by two parameters: permeability (the rate at which a given component is transported through the membrane) and selectivity (the ability to separate in specific way a given component from others).
The transport of different species through a membrane is a non-equilibrium process, and the separation of the different components is due to a differences in their transport rate. In a membrane separation process, the transport rate of a component can be activated by various driving forces such as gradients in concentration, pressure, temperature or electrical potential. In many membrane operations more than one driving force is involved (e.g. pressure and concentration in gas separation, concentration and electrical potential in electrodyalisis, etc.), but all these parameters can be included in one thermodynamic function, the electrochemical potential [1].
Because of their intrinsic properties that well fit the requirements of process intensification strategy, membrane operations have well established applications in many industrial processes [2]. Process intensification is the most interesting strategy available today for realizing a sustainable industrial growth, compatible with a desirable high quality of our life. The strategy of process intensification consists of innovative equipments, design and process development methods that are expected to bring substantial improvements in industrial field, such as reduction of production costs, equipment size, energy consumption, waste generation, and improvment of the remote control, information fluxes and process flexibility.
In this frame, the continuous growth of the modern membrane engineering is an interesting and important case [3].
Some examples of state of the art in membrane technology are reverse osmosis in sea water and brackish desalination, non cryogenic hydrogen and oxygen separation and a significant number of other examples in a variety of industrial areas.
Membrane based artificial organs such as the artificial kidney are a standard part of modern biochemical engineering and medicine. New hybrid artificial organs as the artificial liver, artificial pancreas, are expected to become more and more equally utilized in relatively short period of time and new organs as the artificial retina, or the artificial brain, are attracting the interest of the new generation of membranologists.
Also traditional areas such as encapsulation and packaging will be substantially modified and innovated with the transfer of more basic understanding of transport phenomena and membrane phenomena in general, in these sectors.
The redesigning of overall industrial productions such as the petrochemical plants, by combining various membrane operations suitable for separation and conversion units as integrated membrane systems might become real in few years from now.
Membrane contactors in their various configurations and operations (emulsifiers, crystallizers, strippers, scrubbers, etc.) will make more realistic the opportunities of integrated membrane systems for an industrial sustainable growth [4].
A continuous research work on membrane properties and fundamental aspects of transport phenomena in the various membrane operations is important for the future of membrane science and technology. There is a need for both basic and applied research in order to develop new membranes with improved properties and new membrane processes. These research efforts must take into account the studies done in other areas such as supramolecular chemistry, molecular imprints materials, nano-technology, non linear optics, studies on biological membranes and biological phenomena, etc. More progresses also on anticipating and predicting relationship between membrane chemical properties, their morphology and configuration, with the overall membrane phenomena are still necessary, as also the role of interfacial phenomena and the influence of the properties and phenomena in the solution upstream and downstream the membrane faces .
Biological membranes are able to reproduce themselves continuously, to control important physiological processes, and fouling for this systems does not represent a problem as in artificial systems. The mechanisms which generate our memory or the function of our brain are other at least in part important membrane phenomena. The understanding and transfer of these mechanisms is already creating stimula for the younger generations of membranologists.
Computational strategies will be key tools for the understanding of properties and behavior of materials in order to exploit the potential use of novel highly complex composite systems, new molecules and new multi-functional materials, also in membrane technology.
Membrane characterized by highly selective transport mechanisms, as the perovskite studied for oxygen separation from air, or the palladium for hydrogen purification are suggesting the use of molecular dynamic studies for identifying new structures characterized by similar selectivity toward a larger spectrum of chemical species, or membranes with higher permeability than those already existing.
Molecular dynamics (MD) simulations, for example, predicted that the gas transport inside single-walled nanotubes is orders of magnitude faster than in any other known materials with nanometer-scale pore [5]. This transport, faster than what classical models (Knudsen diffusion) predict, exists because the walls of the nanotubes are much smoother, on the atomic scale, than other materials.
As consequence membrane made of precisely sized nanotubes hold great potential for separation processes. In a recent paper [6] Holt et al. reported about the preparation of double-walled carbon nanotube (less than 2 nm) pores in a silicon nitride membrane. They verified experimentally that the gas and water permeability of these nanotube-based membranes, are several orders of magnitude higher than those of commercial polycarbonate membranes, despite having pore sizes an order of magnitude smaller.
Development of innovative materials with improved properties is a key issue for the further development of the membrane science and technology. Significant progress has been made in the study and realization of new organic and inorganic membranes with controlled structure.
The ability to control pore sizes is an important topic in this frame. Membranes with controlled morphology can be used not only in separation process but also in chemical conversion supplying highly ordered and confined geometries for chemical reactions. The immobilization of catalysts and biocatalysts in their structure appears an interesting new approach for catalyst design [7-8].
Nanoscale control of membrane architecture can further extend the field of application of membrane technology. Nanotechnologies have been used to create membrane structures by the typical methods used for electronic component construction using lasers and etching technique (e.g. track etch membrane) [9].
Self-assembling is another innovative research field in membrane technology. Self-assembly process consists in the regular assembly of small molecular entities into larger supra-molecular structures exhibiting new functions that can not be exhibited by the isolated units [10]. The interaction between subunits are generally due to non-covalent bonds, such as hydrogen bonds, Van der Waals interactions and electrostatic forces.
Membranes applications in sensors and microelectromechanical systems (MEMS) are also increasing of importance.
The development of new device able to give rapid detection of chemical and biological species is central to many areas of life science and industrial production. In particular conducting polymeric materials show major potentiality in this field, and are replacing classical inorganic semiconductor materials because of their better selectivity and rapid measurements, low cost and easy manufacture for their preparation as films [11]. Moreover appropriate molecular design of polymer properties can increase the efficiency of the system.
Membrane technology have enormous potentiality in gas detection, ion selective sensors, biochemical analyses, medical applications, quality control on industrial manufacturing processes, food and beverages. Polymeric membrane, are used in sensor device either participating in sensing mechanism or immobilizing the substance responsible for sensing. Membranes are applied today also for mimic natural sense organs. For example an electronic tongue using membranes based on conducting polymers (polypyrrole and polyaniline) and a lipid-like material components (stearic acid) has been already realized [12].
Very promising can be the use in sensors field of molecular imprinted membrane [13-14], where the memory of a specific substance is imprinted in a polymeric material.
Membranes fabricated using the MEMS technology are finding an increasing number of applications in sensors, actuators and other sophisticated electronic device. However the new area of application of MEMS are creating new materials demands that traditional silicon can't fulfil [15-16]. Polymeric materials, also in this case, are the optimal solution for many applications.
References
[1] Strathmann H., Giorno L., Drioli E., An introduction to membrane science and technology, Pubblisher CNR Roma, ISBN 88-8080-063-9
[2] Drioli E., Fontananova E., Membrane Technology and Sustainable Growth, Chemical Engineering Research & Design 82 (A12) (2004) 1557-1562.
[3] Drioli E., Process intensification using membrane systems. Clean Techn Environ Policy 5 (2003) 3-4.
[4] Drioli E., Curcio E., Di Profio G., State of the art and recent progresses In membrane contactors, Trans IChemE, Part A, Chemical Engineering Research and Design, 83(A3) (2005) 1-11
[5] Sokhan V.P., Nicholson D., Quirke N., Fluid flow in nanopores: Accurate boundary conditions for carbon nanotubes, The Journal of Chemical Physics, 117 (2002) 8531-8539
[6] Holt J.K., Park Y.G., Wang Y., Stadermann M., Artyukhin A.B., Grigoropoulos C.P., Noy A., Bakajin O., Fast Mass Transport Through Sub-2-Nanometer Carbon Nanotubes, Nature 312 (2006) 10341037.
[7] Fontananova E., Donato L., Drioli E., Lopez L., Favia P., d'Agostino R., Heterogenization of polyoxometalates on the surface of plasma modified polymeric membranes, Chemistry of Materials 18 (2006) 1561-1568
[8] Bonchio M., Carraro M., Scorrano G., Fontananova E., Drioli E. Heterogeneous Photooxidation of Alchols in Water by Photocatalytic Membranes Incorporating Decatungstate. Advanced Synthesis & Catalysis 345 (2003) 1119-1126.
[9] Apel P., Track etching technique in membrane technology. Radiation Measurements 4 (2001) 559-566
[10] U.S. Patent No. 6 800 481
[11] Adhikari B., Majumdar S. Polymers in sensor applications. Prog. Polym. Sci. 29 (2004) 699-766.
[12] Riul A. Jr., Gallardo Soto A.M., Mello S.V., Bone S., Taylor D.M., Lattoso L.H.C. An electronic tongue using polypyrrole and polyaniline. Synthetic Metals 132 (2003)109-116
[13] Piletsky S.A., Panasyuk T.L., Piletskaya E.V., Nicholls I.A., Ulbricht M. Receptor and transport properties of imprinted polymer membranes - a review. Journal of Membrane Science 157 (1999) 263- 278.
[14] Donato L., Figoli A., Drioli E. Novel composite poly(4-vinylpyridine)/polypropylene membranes with recognition properties for (S)-naproxen. Journal of Pharmaceutical and Biomedical Analysis 37 (2005) 1003-1008
[15] Huber R., Singer N. Out with the old in with the new. Materials Today 5 (2002) 36-43
[16] Shearwood C., Harradine M.A,. Birch T.S., Stevens J.C. Applications of polyimide membranes to MEMS technology. Microelectronic Engineering 30 (1996) 547-550.
KLAUS-VIKTOR PEINEMANN
Institute of Polymer Research, GKSS Research Centre, 21502 Geesthacht, Germany
klaus-viktor.Peinemann/gkss.de
This lecture will give some insight into the properties of membranes, which are composed from two or more different materials. The first part deals with the description of two component polymeric membranes. The simplest system is the combination of two homogeneous polymers in series or in parallel. The theoretical calculation of mass transfer through these structures is straightforward and reveals no surprises. The selectivity of these structures is always between the selectivities of the single materials. The picture changes, when the different components have pores. Now the selectivity of a two-layer structure might be significantly higher than the selectivity of the individual layers. In addition, selectivities can be achieved, which can never be obtained with pure polymers. If the transport behavior of three layer membranes is analyzed, the flux can be dependant on the sequence of the layers. The flux through a membrane A-B-C (A,B,C are the different layers) can considerably lower than the flux through a membrane A-C-B. It can even be shown, that the addition of a third layer can increase the flux of a two-layer membrane. Besides the theoretical treatment practical examples and applications of the different membrane types will be shown.
Another class of multicomponent membrane structures are "mixed matrix membranes". These membranes are made from polymeric materials, in which either small inorganic particles or low molecular weight organic compounds are dispersed. When designed properly, composite materials can be obtained with the high selectivity of the dispersed phase and the mechanical properties of the polymeric phase. One of the fundamental questions of the mixed matrix concept is how the permeability of the polymer should match with the permeability of the dispersed phase. The mathematical treatment of material transport through mixed matrix membranes is more complex than transport modelling through multilayer membranes. The following equation can be obtained for the selectivity of a mixed matrix membrane [1]
 |
Where ![]() is the effective selectivity of the mixed matrix
membrane,
is the effective selectivity of the mixed matrix
membrane, ![]() is the permeability ratio of continuous phase to
dispersed phase for the fast component and
is the permeability ratio of continuous phase to
dispersed phase for the fast component and ![]() and
and ![]() are the selectivities of the continuous and dispersed phase,
consequences of this result and future perspectives of mixed matrix
membranes will be discussed.
are the selectivities of the continuous and dispersed phase,
consequences of this result and future perspectives of mixed matrix
membranes will be discussed.
An smart way to manufacture structured multicomponent materials for separation is the self-organisation of block-copolymers. Block copolymers are a fascinating class of polymeric materials, since they offer the possibility to combine different properties along one macromolecular chain. The morphological features of block copolymers, especially tailor-made diblock, triblock and higher block copolymers of different chain topologies has been an increasingly intense research subject during the last decades. Block copolymers composed of chemically different chain sequences tend to segregate on an intramolecular level, if there are repulsive interactions between dissimilar blocks. Due to the connectivity of the blocks a macroscopic phase separation is suppressed, and instead a so-called microphase separation on the length scale of the macromolecular chains occurs, which leads to a self-assembly into different microphase morphologies, which depend on the chemistry, degree of polymerization, composition [2] and also often on the way of preparation of thin film material [3].
Due to their defined and tunable morphology block copolymers play an important role for applications where selective material transport is essential (e.g. food packaging, controlled drug release, breathable but watertight clothing, industrial gas separation). The current status of membrane formation by self-assembly of block copolymers will be discussed.
The lecture will conclude with a discussion of the preparation and application of stimuli responsive membrane materials for different applications, e.g. for the development of an artificial pancreas.
[1] Klaus-V. Peinemann, Membranes for Gas Separation, in: Suzana P. Nunes, Klaus-V. Peinemann (Eds), Membrane Technology in the Chemical Industry, 2nd Ed., Wiley-VCH, Weinheim, 2006
[2] L. Leibler, Macromolecules 1980, 13, 1602
[3] H. Elbs, C. Drummer, V. Abetz, G. Krausch, Macromolecules 2002, 35, 5570
E. MARAND, S. KIM, B. VAUGHAN
Department of Chemical Engineering, Virginia Polytechnic Institute and State University, 138 Randolph Hall, Blacksburg, VA 24061, USA
Separations represent a significant component in the petroleum and chemical industry. In particular, there is a strong interest in membranes, which can potentially handle large-scale separations such as recovery of H2, natural gas purification, O2/N2 separation, and a variety of isomer and hydrocarbon separations [1,2,3]. The inherent advantages of membranes such as modular design and simple and environmentally safe operating conditions offer modern alternatives to traditional gas and vapor separation processes such as pressure swing adsorption and cryogenic distillation.
The vast majority of commercial membranes are polymeric because of processing feasibility and cost. Unfortunately, the performance of polymeric membranes is governed by a fundamental trade-off between selectivity and flux [4,5] demarcated by "Robeson's upper bound ". This limitation and the need for robust membrane systems have driven research towards development of new materials in the last decade [1-3, 6-17]. One promising direction has focused on nano-composite materials in which polymers act as supporting matrices for other high performance materials that typically cannot form membrane films on their own [6-17]. Ideally, the second material in a composite membrane should have excellent properties as a gas adsorbent or a molecular sieve, be readily available in particle morphologies suitable for dispersion in a polymer film of sub-micron thickness, and form high quality interfaces with the polymeric matrix. In our studies we have examined a variety of filler particles ranging from zeolites, mesoporous silica, aluminophosphate flakes to carbon nanotubes. In this presentation we will highlight some of the key finding associated with the membrane performance of these nano-composite systems.
Since the discovery of the M41S family of mesoporous molecular sieves by Kresge et al.,18,19 these materials have received widespread interest as catalysts, adsorbents and membranes because of their high surface areas, tunable pore sizes (2-50 nm) and surface chemistry via functionalization. The surface of mesoporous silica is decorated with reactive silanol groups, which can be used for surface modification to introduce favorable interactions with polymers 20-24. In this study, we have focused on incorporating MCM-48 silica in a polysulfone mixed matrix membrane. TheMCM-48 was synthesized by a templating method and characterized it with X-ray diffraction (XRD), pore size analysis, and field emission scanning microscopes (FESEM). The particles are on the order of 1 micron. Helium permeation data and SEM images of as-synthesized MCM-48/PSF MMMs suggest that MCM-48 silica particles adhered well to PSF and prepared MMMs were defect free. As shown in Table I, mesoporous MCM-48 materials offer the favorable effect of large increase in gas permeability in MMMs without sacrificing selectivity.
Table I. Gas permeabilities (Barrer) of various gases in the pure polysulfone and MCM-48 MMMs
|
Membrane |
MCM-48 wt % |
He |
CO2 |
O2 |
N2 |
CH4 |
|
PSF |
0 |
8.02±0.19 |
4.46±0.10 |
0.98±0.07 |
0.18±0.01 |
0.17±0.01 |
|
MCM48/PSF |
10 |
15.75±0.53 (96.38%)a |
8.45±0.13 (89.46%) |
1.84±0.10 (87.76%) |
0.32±0.02 (77.78%) |
0.33±0.02 (94.12%) |
|
MCM48/PSF |
20 |
32.10±0.83 (300.25%) |
18.21±0.41 (308.30%) |
4.14±0.01 (322.45%) |
0.77±0.02 (327.78%) |
0.77±0.02 (352.94%) |
a ( ) increment from pure polymer
The continuous pathways present in the polymer matrix with the high loading of MCM-48 silica allow the gas molecules to diffuse solely through the molecular sieve phase, thus improving membrane performance. This performance can be enhanced even further by employing much smaller mesoporous silica particles on the order of 20 to 50 nm, which can be incorporated at larger volume fractions and into thin selective layers. Some preliminary results using nanosized mesoporous silica are shown in Table II. The observed increases in both the diffusivity and solubility make mesoporous silica an attractive additive for enhancing the gas permeability of low permeability polymers.
Table II. Gas permeabilities (Barrer) of various gases in the pure polysulfone and nano-sized (~30 nm) MCM-41 MMMs
|
Membrane |
Mesoporous silica wt % |
He |
CO2 |
O2 |
N2 |
CH4 |
|
PSF |
0 |
8.02±0.19 |
4.46±0.10 |
0.98±0.07 |
0.18±0.01 |
0.17±0.01 |
|
MCM41/PSF |
20 |
16.25±0.07 (102.62%)a |
7.59±0.14 (70.18%) |
1.67±0.01 (70.41%) |
0.30±0.00 (66.67%) |
0.31±0.00 (82.35%) |
|
MCM41/PSF |
30 |
46.02±0.21 (473.82%) |
22.93±0.20 (414.26%) |
5.01±0.21 (411.22%) |
0.98±0.05 (544.45%) |
1.02±0.00 (500.00%) |
a ( ) increment from pure polymer
While mixed matrix membrane systems such as those described above enhance permeability without limiting selectivity, recent research efforts have also focused on size selective systems. According to a permeable flakes model developed by Cussler25, the ideal geometric form for a selective filler material is a very thin layer (i.e. less than 100 nm) with large aspect ratios. Thin separating layers are desirable for high productivity or flow rates and for incorporation into hollow fiber membranes. According to Cussler25, if the flakes are highly selective, the relative fluxes should be proportional to the square root of the inherent selectivity. Achieving this selectivity requires matching the diffusion coefficient in the polymer continuum to the geometric average of the diffusion coefficients in the flakes times the aspect ratio of the flakes.
We have fabricated polymer/selective flake nanocomposite membranes using various polymers and a porous layered aluminophosphate. The aluminophosphate, AlPO, contains sheets with the 2-D net structure defined by 4, 6, and 8 membered rings (rings of interconnected AlO4 and PO4 tetrahedra)26 as shown in Figure 1.
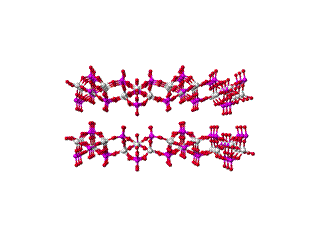
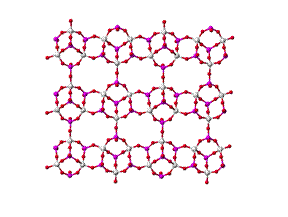
Figure 1 Crystalline structure of AlPO; side and top views
The large opening in the AlPO 4 ´ 6 ´ 8 net is an 8MR with an estimated opening of ca. 4.44 ´ 3.28 ´ 3.17 Å. The fabrication of the mixed matrix membranes requires initial separation of the AlPO layers in order to achieve intercalation of the polymer. This has been attempted, both, by using an ammonium-based surfactant to swell the layers and by employing in-situ polymerization with the purpose of first incorporating the smaller monomer, which is subsequently polymerized. Mixed matrix membranes made with AlPO typically demonstrated lower permeabilities over the pure polymer, particularly in the case of larger gas molecules such as CH4, O2 or N2. This suggests that the pore openings in the AlPO sheets are not as accessible as originally believed. Methods for removing excess surfactant are being investigated.
Another approach that may have the potential to improve both permeability and selectivity of mixed matrix materials is the incorporation of oriented carbon nanotubes in polymer membranes. Molecular simulation and atomistic studies of single walled carbon nanotubes (SWNTs) predict that SWNTs have both high selectivities and very high fluxes for gas transport 27,28. Recently, we have fabricated nanocomposite membranes using polysulfone as the polymer matrix and isotropic as well as oriented carbon nanotubes. Results of preliminary permeability measurements of several simple gases in the carbon nanotube/polymer nanocomposite systems indicate that the open ended, aligned, carbon nanotubes can significantly enhance the permeabilities of gases through the membrane. Data, shown in Table III, compares the permeabilities of various gases through the pure polysulfone and nanocomposite membranes having 4 vol % randomly oriented CNTs (r-CNT/PSF) and 2.0 vol% oriented CNTs (o-CNT-PSF). Orientation enhances the accessibility of the gases to the pores. Fig. 2 shows the SEM image of the oriented multi-walled carbon nanotubes on a quartz support that were used in the manufacture of the membrane system. Because these multi-walled carbon nanotubes have larger pore diameters than single walled carbon nanotubes, they did not produce a molecular sieving effect. This is demonstrated in Table IV, where the selectivities can be primarily attributed to those of the polymer. However, we expect that the inclusion of small-diameter single walled carbon nanotubes in the polymer matrix will enhance the gas selectivities. This work is on-going.
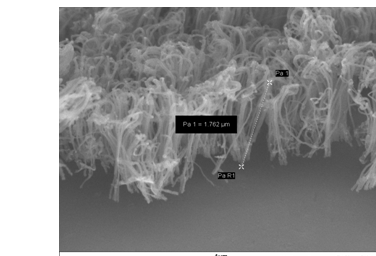
Figure 2 Oriented Multi-walled Carbon Nanotubes created by CVD
(Sample from Prof. Gordon Wallace, IPRI, University of Wollongon, Australia)
Table III. Gas permeabilities (Barrer) of
simple gases in pure polysulfone and carbon nanocomposite membranes.
[![]() ]
]
|
Membrane |
vol % CNTs |
He |
CO2 |
O2 |
N2 |
CH4 |
|
PSF |
0 |
7.88±0.01 |
3.90±0.05 |
0.84±0.00 |
0.17±0.00 |
0.17±0.00 |
|
r-CNT/PSF |
4.0 |
10.20±0.56 |
5.12±0.04 |
1.16±0.00 |
0.23±0.00 |
0.27±0.00 |
|
o-CNT/PSF |
2.0 |
21.16±0.17 |
10.06±0.15 |
2.15±0.01 |
0.43±0.04 |
0.42±0.00 |
Table IV Gas selectivities for polysulfone and nanocomposite membranes
|
Membrane |
vol % |
He/CO2 |
CO2/CH4 |
O2/ N2 |
CH4/ N2 |
|
PSF |
0 |
2.01 |
23.55 |
5.07 |
1.00 |
|
r-CNT/PSF |
4.0 |
2.10 |
23.95 |
5.04 |
1.17 |
|
o-CNT/PSF |
2.0 |
2.00 |
23.95 |
5.00 |
0.98 |
References
F. BOSC, A. AYRAL * and A. JULBE
Institut Européen des Membranes, UMR n° 5635 CNRS-ENSCM-UMII, CC047,
Université Montpellier II, Place Eugene Bataillon, 34095 Montpellier
Cedex 5, France (*Corresponding author: Andre.Ayral/iemm.univ-montp2.fr)
The latest developments in ceramic membranes are closely related to
recent advances in materials science l,2,
in particular in the development of nanomaterials by innovative sol-gel
or hydrothermal routes. In correlation with chemical engineering and
transport modeling considerations, several complementary strategies can
be adopted in term of material engineering. The first one is the
selection of the most suitable solid phase to manage the fluid-membrane
interactions. Layers exhibiting specific physical or chemical
properties can be advantageously prepared. Multifunctional membranes
coupling separation with an other functionality like catalysis,
photocatalysis or adsorption can also be designed. A second aspect
deals with the tailoring of the nanoporous texture. The third point is
the design of the membrane shape to increase the surface-to-volume
ratio, to promote anti-fouling and hydrodynamics properties. These new
approaches in the design of ceramic membranes whose properties are
tailored in agreement with the requirements of the final applications
will be illustrated.
The optimisation of the fluid-solid interactions using nanoporous or
dense ceramic materials with adapted chemical and physical properties
is a key-point for the improvement of the separative properties of
ceramic membranes. It is also essential for multifunctional membranes
with additional functions like catalysis, photocatalysis or adsorption.
Nanofiltration is a membrane process involving microporous
membranes. Different types of single or mixed oxide ceramic nanofilters
have been prepared from aqueous or organic sols. Due to the amphoteric
behavior of the used oxides, the separative properties of these ceramic
nanofilters for ionic solutes in aqueous solutions will depend on both
sieving and electrical effects. Each oxide exhibits intrinsic values of
zero point of charge and isoelectric point associated with specific
conditions. Application of membrane processes to non-aqueous liquids
appears as very promising. Ceramic membranes offer utmost advantage
exhibiting a very good stability with practically all organic solvents
and in a wide temperature range. The first results obtained with NF
ceramic membranes show that permeation of organic solvents does not
simply obey conventional Darcy's law 3.
Dense metallic membranes have been extensively studied for the
selective transport of H2 or O2 4.
However, dense oxide ceramic membranes are the most attractive in term
of durability and reliability. Transport of O2 or H2
occurs via the conduction of the ions O2- or H+
through the oxide network. A possible strategy is to design a composite
membrane adding an electron conductor as a second phase. In the case of
the external circuit, a voltage can be applied by an electric generator
to favor O2 transport (oxygen pump). Several oxides (e.g.
perovskites) exhibit mixed ions-electrons conduction. In parallel to
the concept of fully dense membrane, application of ion conducting
mesoporous membranes has been recently considered 5. A
synergetic effect of the Pd or Pt metallic nanoparticles on oxygen
transport has been evidenced, in relation with the triple phase
boundary concept. This is a potential direction to be
investigated for membrane applications requiring higher fluxes and
lower selectivity than dense membranes.
Coupling two operations like membrane separation and catalytic
reaction or adsorption in a given process of synthesis, purification or
decontamination of effluents is intrinsically interesting from a
general technical-economical point of view. Ceramic membranes are ideal
solid-fluid contactors which can be efficiently used to couple
separation and heterogeneous catalysis for membrane reactor
applications 6.
The concept of combining membranes and reactors is being explored in
various configurations, which can be classified in three groups,
related to the role of the membrane in the process. The membrane can
act as: an extractor: the removal of product(s) increases the
reaction conversion by shifting the reaction equilibrium; a
distributor: the controlled addition of reactant(s) limits side
reactions and finally an active contactor:
the controlled diffusion of reactants to the catalyst can lead to an
engineered catalytic reaction zone. The different types of membrane
reactor configurations can be also classified according to the relative
placement of the two most important elements of this technology: the
membrane and the catalyst. Three main configurations can be considered:
the catalyst is physically separated from the membrane; the catalyst is
dispersed in the membrane or the membrane is inherently catalytic.
A number of semi-conducting single or mixed oxides like TiO2
exhibit photoactivity under UV-visible irradiation. Several papers have
been published in the past concerning the use of membranes in
photocatalytic reactors. In most cases, the membranes do not present
photoactivity; they are used to separate reactants and to retain the
titania particles dispersed inside the reactor loop. It is interesting
to develop intrinsically photoactive ceramic membranes for membrane
reactor, antifouling or VOCs elimination7. Ceramic membrane
with adsorptive properties is a new field of application for
multifunctional membranes. As an example, ZnO membranes can be used for
removing hydrogen sulfide, H2S by chemisorption 8-10.
Tailoring of the porosity is very important because the porosity,
pore size distribution, connectivity and tortuosity of the pore network
are parameters which define both the permselectivity and the
permeability of the porous membranes. With conventional sol-gel routes,
the pore size distribution is usually broad and the tortuosity is
important with the presence of constrictions. Thus ordered
interconnected pore networks with a constant pore size, are strongly
attractive. Hierarchical porosity and adaptive porosity are also
fascinating approaches to increase or manage the permeability of
ceramic membranes.
Zeolites are ultramicroporous solids with a structural porosity.
Zeolites exhibit a set of attractive properties including regular pore
sizes with molecular dimensions (enabling shape or size selective
catalysis or separation), a high thermal stability, acid or basic
properties, hydrophilic or organophilic properties, possibility of ion
exchange, dealumination-realumination, isomorphous substitution and
insertion of catalytically active guests (transition-metal ions,
complexes or chelates, basic alkali metal or metal oxide clusters and
enzymes). The specific properties of zeolites coupled with the
separation properties of membranes open the field to a great area of
exiting research for the future 11,12. In spite of the
progress made in the field of supported zeolite membranes during the
last decade, a number of points still need to be explored or further
studied such as: synthesis control and reproducibility, control of
membrane thickness and location (at the surface or in the support
pores), control of membrane quality, detection of microdefects and
influence on membrane performance, control of zeolite crystal
orientation,
extension of membrane synthesis to zeolite structures with smaller
pores (e.g. 3Å), modification of existing membrane performance (ion
exchange, plugging defects, insertion of catalytically active guests).
The main problem remains to reproducibly obtain a continuous filtering
barrier with a high permselectivity. In order to overcome these
problems, a promising strategy has recently been considered: the
pre-seeding/secondary growth method (Fig.l) 13. When
coupled with microwave (MW) heating, the method leads to homogeneous
membranes within a few hours 14.
|
|
|
|
|
(a) |
(b) |
(c) |
Fig.l SEM observations of αAl2O3
support (200 nm pore size) covered with (a) a
layer of MW derived silicalite-1 seeds, (b) a MFI membrane formed by
MW-
assisted secondary growth (150°C, 2h, 400 W) from a layer of MW
derived
silicalite-1 seeds, (c) SOD membrane formed by classical secondary
growth (95°C,
12h) from a layer of MW derived SOD seeds.
Extension of the molecular sieves to the mesoporosity range is
possible using lyotropic liquid crystal mesophases as removable
templates. These mesophases result from the self-assembly of
surfactants or amphiphilic molecules and can be thermally or chemically
eliminated after the formation of the inorganic network. This approach
enables the preparation of materials exhibiting an ordered mesoporosity
with pores usually ranging from two to more than ten nanometers. Since
the first articles15, many investigations were carried out
on this new class of materials, in particular for the preparation of
sol-gel derived silica layers exhibiting hexagonal, cubic or lamellar
structures using cationic surfactants of alkyltrimethylammonium halide
type 16,17. At the same time, this synthesis method
was extended to the use of non-ionic surfactants 18 and
block copolymers 19. The preparation of membranes with
other mesoporous oxides was also investigated 2,7.
It can be advantageous to generate extraporosity at a larger scale
in the separative layer. The main condition which has to be respected
is that the additional porosity must not be directly interconnected in
order to preserve the cut-off fixed by the porosity of the continuous
phase (Fig.2). Moreover, the presence of removable porogens inside the
starting suspensions modifies their rheology and decreases their
ability to infiltrate the porous substrates. It can be used to reduce
the number of intermediate layers of the asymmetric membranes.
Templating by polystyrene latex was used to produce individual
macropores inside silica layer, or other oxides (Fig.2).
|
|
|
|
|
|
Isolated spherical macropores |
Isolated mesopores |
Nanocrystalline anatase walls with Interconnected micropores |
|
|
Fig. 2 Photocatalytic anatase membrane with a hierarchical
porosity 20. |
|||
An innovative method has been proposed to better regulate the flux
in the reactor: the concept is based on the use of a chemical valve
membrane whose permeability could be controlled by the red/ox
properties of the gas phase (Fig. 3) 21.
|
|
|
||
|
(a) |
(b) |
||
|
Fig. 3: Schematic representation of the O2 profile
generated in a tubular reactor: (a) Classical inert uniform porous
membrane and (b) 'Chemical valve' membrane. |
|||
Numerical simulations and experiments with turbulence promoters or
pulsating flux confirm that promoting turbulence close to the membrane
surface can partially overcome damageable phenomena like fouling and
concentration polarizations in tangential filtration processes. An
original approach has consisted in the design and preparation of
tubular ceramic membranes with helical relief stamps. Permeation
measurements demonstrated the interest of such geometries compared to
conventional smooth membranes 22.
Due to their high stiffness and brittleness, it is not possible to
extend to ceramic membranes all the geometries applicable with organic
ones, like spirals, which give rise to high surface to volume ratios. A
very attractive type of membrane is the ceramic hollow fiber with a
external diameter of less than 1 mm and ceramic walls with a thickness
of few hundreds μm. An increasing number of papers have been published
during the last decade on the preparation and applications of such
ceramic hollow fibers 23-25. Different types of mesoporous,
microporous or dense separative layers have been deposited usually on
the outer surface of macroporous hollow fibers like γ-alumina 26,
titania 27 or zeolite 28 layers. Other
types of multifunctional miniaturized devices using ceramic hollow
fibers should be developed in the next future2.
Multifunctional and adaptive membranes, miniaturized and integrated
separative devices are current trends in membrane science research and
development. Increasing societal requests in terms of environment
protection, health and energy saving are long term driving forces for
such activities.
Taking benefit of the advances in materials science, it is possible
to prepare ceramic-based membranes with improved properties, with
regard to the specifications of the aimed final applications. The
choice of the materials, the tailoring of the porosity and the shape of
the membrane are adaptable variables at the disposal of the designers.
1. L. Cot, A. Ayral, J. Durand, C. Guizard, N. Hovnanian, A. Julbe
and A. Larbot, Solid State Sciences 3 [2] (2000) 313-334.
2. A.Ayral A. Julbe and C. Guizard, Chemical Processing of
Ceramics edited by B. Lee and S. Komarneni (CRC Press, Boca Raton,
2005) pp. 629-666.
3. C. Guizard, A. Ayral and A. Julbe, Desalination 147
(2002) 275.
4. V.M. Gryaznov et al., Rus. J. Phys. Chem. 47 (1973) 1517.
5. C. Guizard, C. Levy, L. Dalmazio and A. Julbe, Mat. Res. Soc.
Symp. Proc. 752 (2003) 131.
6. A. Julbe, D. Farrusseng and C. Guizard, Journal of Membrane
Science 181 (2001) 3.
7. F. Bosc, A. Ayral and C. Guizard, Journal of Membrane Science
265 (2005) 13.
8. R. Goswamee, F. Bosc, D. Cot, A. El Mansouri, M. Lopez, F.
Morato, A. Ayral, Journal of Sol-Gel Science and Technology 29
(2004) 97.
9. J. Gwak, A. Ayral, M. Komaki, C. Nishimura, Transactions of
The Materials Research Society of Japan 30 (4) (2005) 991.
10. L. Nazsalyi, F. Bosc, Z. Hórvölgyi and A. Ayral in Proceedings
of the ICIM9, June, 26-29 2006, Lillehammer, Norway.
11. J. Coronas and J. Santamaria, Sep. Purif. Meth. 28
(1998) 127.
12. A. Julbe, in J. Cejka and H. Van Bekkum (Eds), Studies in
Surface Science and Catalysis, No. 157, Elsevier, Amsterdam, 2005,
pp: 135-160.
13. Z. Lai, G. Bonilla, I. Diaz, J. G. Nery, K. Sujaoti, M.A. Amat,
E. Kokkoli, O. Terasaki, R.W. Thompson, M. Tsapatsis, D.G. Vlachos, Science
100 (2003) 456.
14. J. Motuzas, A. Julbe, R.D. Noble, A. van der LEE, Z.J.
Beresnevicius, Mesoporous and Microporous Materials 92 [1-3]
(2006) 259-269.
15. C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck,
Nature 359 (1992) 710.
16. A. Ayral, C. Balzer, T. Dabadie, C. Guizard and A. Julbe, Catalysis
Today, 25, 219-224 (1995).
17. M. Klotz, A. Ayral, C. Guizard and L. L Cot, Journal of
Materials Chemistry, 10 (2000) 663.
18. P.T. Tanev and T.J. Pinnavaia, Science, 267 (1995) 865.
19. D. Zhao, Q. Huo, J. Feng, B.F. Chmelka and G.D. Stocky, J.
Am. Chem. Soc., 120 (1998) 6024.
20. F. Bosc and A. Ayral in Proceedings of the ICIM9, June,
26-29 2006, Lillehammer, Norway.
21. D. Farruseng, A. Julbe, C. Guizard, Journal of the American
Chemical Society 112 (2000) 12592.
22. L. Broussous, P. Schmitz, H. Boisson, E. Prouzet and A. Larbot, Chemical
Engineering Science 55 (2000) 5049.
23. H.W. Brinkman, J.P.G.M. van Eijik, H.A. Meinema and A. Terpstra,
Bulletin American Ceramic Society, 78 (1999) 51.
24. S. Liu, X. Tan, K. Li and R. Hughes, J. Membrane Science
193 (2001) 249.
25. X. Xu, W. Yang, J. Liu, L. Lin, N. Stroh and H. Brunner, J.
Membrane Science 229 (2004) 81-101.
26. Smid, C.G. Avci, V. Gilnay, R.A. Terpstra and J.P.G.M. Van Eijk,
J. of Membrane Science 112 (1996) 85.
27. S. Liu and K. Li, J. Membrane Science 218 (2003) 269.
28. X. Xu, W. Yang, J. Liu, L. Lin, N. Stroh and H. Brunner, J.
Membrane Science 229 (2004) 81.
I. GENNE, E. BRAUNS, W. DOYEN
VITO, Flemish Institute for Technological Research, Environmental and Process Technology, Boeretang 200, B-2400 Mol, Belgium (inge.genne/vito.be, http://www.vito.be )
1. Introduction
Membrane filtration is a wide and fast growing market. Although the applications of ceramic membranes are increasing, the wide majority of the membrane market is still owned by the polymeric materials. To outsiders, the synthesis of polymeric membranes can look surprisingly simple but for membrane researchers and polymer scientists the formation process is a very complex and intriguing phenomenon.
For porous membranes, separation occurs based on differences in sizes, shape and charge properties while for non-porous or dense membrane sorption and diffusion are important. Besides the membrane structure, also the material properties of the membrane itself are very important. The strong link between material, structure and operation implies that for a large number of filtration applications a broad variety of membranes is required. Nowadays, the knowledge of polymeric systems that lay at the foundation of the membrane formation process is evolved in such a way that it is possible to synthesize 'tailor-made' membranes adapted to specific applications.
During the last decade, the membrane filtration market has been characterised by numerous, successive takeovers. Smaller membrane producers with innovative membranes have been bought by bigger multinationals to increase their product range. Simultaneously, pressure driven membrane filtration techniques have found their way to the market of large scale water treatment applications. As economic interest and competition grows, details on membrane composition and manufacturing methods are regarded as proprietary information by most suppliers. For membrane researchers the challenge is to get to the bottom of the synthesis routes and to really develop a feeling with the polymer science. Research on the formation processes is focused on innovative membrane concepts and further improvements of one or more of the key features: permeability, selectivity, fouling properties, mechanical strength and chemical stability.
To discuss the different membrane types and materials, a distinction is made between the low pressure applications, micro-and ultrafiltration, and the high pressure applications, reverse osmosis and nanofiltration.
2. Micro- and ultrafiltration membranes
Most polymeric filtration membranes are prepared by the so called phase-inversion technique. The phase-inversion process makes use of the physical-chemical properties of polymer solutions. This means that under certain conditions of composition and temperature, a polymer solution will become thermodynamically unstable and liquid-liquid phase separation will occur. When this process is followed by a solidification process (gelation, crystallization or glass transition), phase-inversion takes places. In practice, the phase inversion is usually induced by bringing the polymer solution in contact with a non-solvent or a vapor stream. The formation of asymmetric structures is induced by of kinetic effects, controlled by the rates of diffusion in and out the polymer film.
Even a small variation in formation parameters makes it possible to turn the same polymer solution into a wide variety of structures. Figure 1 shows two examples of asymmetrical structures resulting from the same polymer-solvent system. The high sensitivity of the formation process enhances a large flexibility but also makes the reproducibility of the process more difficult.
Depending on the envisaged application, the membranes are cast as flat sheets on a support or spun into self-supporting capillaries. By controlling the phase-inversion process, the separating layer or skin can be created on the inside, the outside or on both sides of the membrane.


a b
Fig. 1 Cross-section of capillary UF membrane with typical asymmetric structure, a) sublayer with finger-shaped pores b) sub layer with sponge-like pores
Aiming for a broad range of applications, the preference goes to membranes with a good chemical stability and a high temperature resistance. A stable polymer is very hard to process because it can not be solved in traditional solvents. Therefore, material selection always comes down to finding a compromise between good stability and ability to process.
For the processing of semi-crystalline polymers like Teflon (PTFE), polypropylene (PP) or polyethylene (PE) membrane synthesis occurs by the stretching technique. These polymers are used because of their high thermal and chemical stability and are very hard to dissolve,. During this synthesis process, the polymer film is stretched perpendicular to the crystalline orientation, resulting in highly porous structures.
The first generation of MF/UF membranes was prepared from cellulose acetate (CA). Nowadays, the majority of MF/UF membranes for filtration applications are prepared of polyethersulphone (PES), polyacrylonitrile (PAN), polyvinylidenefluoride (PVDF), polypropylene (PP) or polyethylene (PE).
All these polymers can be formed, through specific manufacturing techniques, into membrane materials having desirable physical properties. All these polymers have reasonable chemical resistance but they are also hydrophobic which decreases their affinity for water and makes them susceptible to fouling by hydrophobic matter. Hydrophilic materials like CA are less susceptible to fouling but the application is limited due to restricted pH stability.
To reduce the fouling properties of hydrophobic membrane polymers normally necessitates blending (e.g. with polyvinylpyrrolidone) or surface modification of the base material to produce a hydrophilic surface (plasma treatment, grafting, chemical reaction…). The modified membranes with permanent hydrophilic surfaces are put on the market as so called 'low-fouling' membranes.
Compared to the CA membranes, the use of the second generation of polymers has also led to an optimization of the membrane structure, displayed by an improvement in porosity and pore connectivity. The 'low-pressure UF' membranes are characterized by higher permeabilities and corresponding lower operating process pressures.
During the last couple of years, specific effort has gone not only to the membranes physical and chemical properties but also to improve its mechanical characteristics. During operation of large scale capillary modules, severe down-time and high operational costs were caused by capillary breakage. One of the examples of recent developments to increase mechanical strength is shown in Figure 2. The membrane is configured as a thick capillary (outer diameter of 4.2 mm) with multiple channels (9 channels with inner diameter of 0.7 mm).
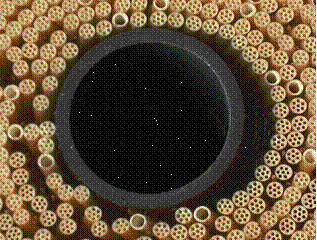

Fig. 2 Cross-section of Multibore capillary membranes
Another need for increased mechanical strength resulted from the
development of the innovative technology of 'submerged' MF/UF membrane
applications. For these type of applications, flat sheet or capillary
membranes are constructed without module housing and they are directly
immersed in the feed compartment. This concept can be applied for MF/UF
water treatment or for membrane bioreactors (MBR). These membranes have
to be able to withstand continuous movements and to reduce fouling,
there is a demand for backflush cleaning possibilities. For these
applications, new types of 'composite' membranes were developed with
integrated supporting materials that were put in during synthesis.
3. Reverse osmosis and nanofiltration membranes
Dense RO membranes are characterized by a good retention under condition that the solubility-diffusion mechanism for the components of the feed is secondary to the water flux through the membrane. Polymer selection therefore occurs on the basis of solubility parameters.
Similar to MF/UF, the first generation of RO membranes was made from CA. An important development for the RO process was the creation of the "thin film composite" structures (TFC). The term composite refers to the two layer structure composed of a very thin toplayer (0.2 µm), functioning as separation layer, chemically bounded to a supporting layer. The supporting layer consists usually of a PSf UF membrane. Almost all commercial membranes have a polyamide (PA) toplayer. The major drawback of the PA material is its limited resistance to chlorine.
The TFC membranes have a higher flux and a better selectivity than the CA membranes and can be operated at lower pressures. The so-called ultra low pressure TFC membranes can be operated at pressures below 10 bar and have a flux of 15-40 l/hm².
NF membranes are porous, causing the separation of uncharged
particles by the sieving mechanism, similar to MF/UF membranes. The
composition of NF membrane is similar to RO membranes but with a more
loose network structure. Their ion-selectivity can be generated by
building fixed charged groups
(-COOH, -SO3H) at the surface or inside the structure of the
NF membrane.
To further improve the NF membrane properties, three layered membranes were developed, introducing an intermediate layer with specific properties. The application of this extra layers lead to a decrease of the surface roughness and reduces the fouling tendency.
A lot of research efforts and new developments are focused on the increase of the NF membrane stability at low pH and at high temperatures.
4. Conclusions
Since the first polymeric membrane in 1974, a lot of research on membrane materials and synthesis techniques took place. Major developments have lead to better performing membranes which are versatile to a broad application field. For several applications it is already so that the application filed for the membranes is not restricted by the membrane material itself but by the housing or the side equipment. Research efforts will continue to further optimize the structure, developing 'tailor made' filtration membranes, with an emphasis on the improvement of the chemical and mechanical resistance of the materials.
In parallel, intelligent combinations of existing and new materials, each with their characteristic properties, will lead to the development of new materials with unique properties. Already now, research on hybrid materials takes place by combining polymers with catalysts, zeolites or nanoparticles.
In all these areas, improvements and innovations are required to expand the application filed and really fine -tune the desired membrane properties. The synthesis of polymeric membranes is not only science but can also be considered as an art.
Enrico Drioli, Gianluca Di Profio
Institute on Membrane Technology (ITM-CNR), c/o University of Calabria, Via P. Bucci, 17/C, 87030, Rende (CS), Italy
Department of Chemical Engineering and Materials, University of Calabria, Via P. Bucci, 87030, Rende (CS), Italy.
Tel.: +39 0984 492039; Fax: +39 0984402103; E-mail:
e.drioli/itm.cnr.it
Since process intensification was considered one of the most promising strategies in the framework of an industrial sustainable development, membrane technology was established as powerful tool working in this direction. In this context, membrane operations have the potential to replace conventional energy-intensive techniques (i.e. distillation and evaporation), to accomplish the selective and efficient transport of specific components, to improve the performance of reactive processes and, in ultimate instance, to provide reliable options for a sustainable industrial growth. The possibility to realize compact membrane systems able to perform the same standard unit operations of the chemical industry - and of any other industrial process involving molecular transformations and separations - is becoming nowadays reliable, driven by the incentive to revolutionize the traditional concept of heat exchangers, pumps, compressors, absorbers, extractors, evaporators, etc. Moreover, the redesign of important production cycles by combining various membrane operations available in the separation and conversion units emerges as reliable and attractive opportunity due to the synergic effects that can be reached. Among the large variety of membrane operations, Membrane Contactors (MCs) represent relatively new membrane-based devices that, because of their potential advantages, are gaining consideration both in industrial and scientific fields. In particular, the reliability of membrane contactors technology is today increasing in a large part of industrial processes where traditional membrane separation units, such as Reverse Osmosis, Microfiltration, Ultrafiltation, Nanofiltration, Electrodialysis, Pervaporation, etc., are already routinely employed.
Membrane Contactors represent a technology in which porous membranes are used as tools for inter-phase mass transfer: in synthesis, the membrane does not act as a selective barrier, but the separation is based on the principles of phase equilibrium. All traditional stripping, scrubbing, absorption, and liquid-liquid extraction operations, as well as emulsification, crystallization, phase transfer catalysis, can be carried out according to this configuration.
Moreover, the integration of MCs with other traditional membrane operations makes them very competitive against conventional unit operations, and able to meet all the requirements of process intensification concept. An overview on the state of art and on the recent developments in MCs technology is here presented. Particular emphasis is addressed to innovative MCs applications, such as Membrane Emulsification, Membrane Crystallization, and Phase Transfer Catalysis, which are recently emerging as alternative options in important industrial sectors.
Traditional carrier-mediated membrane contactors systems like Liquid Supported Membranes show today an increased stability and efficiency in separation processes, and fixed sites membranes seem to offer interesting perspectives for utilizing the carrier mediated transport technique in the treatment of hydrometallurgical solutions. In gas/liquid contactor applications, membrane absorbers and strippers already represent well assessed techniques with important applications in microelectronic and nanotechnology industry; moreover, they assure a controlled extraction of dangerous, harmful, or corrosive gasses in agro-food, pharmaceutical, or in petrol-chemical fields. Membrane crystallizers are today opening new perspectives for a better control of the final particulate product: apart from inorganic salts and small organic compounds, membrane crystallization is a promising technique also in macromolecular crystallization.
Membrane emulsification processes have potential to produce monodisperse emulsions and micro- to nano-sized droplets with shear sensitive components: disperse phase flux, wetting properties, pore size, porosity, and pore shape are membrane structural properties that control the average droplet size and their distribution. As direct consequence, drug delivery and encapsulated catalysts systems have increased in reliability in phase transfer catalysis applications.
The performances of MCs strongly depend on the properties of the membranes used. In general, high hydrophobicity is required in order to prevent wetting and mixing between contacting phases; elevated porosity leads to high fluxes, but might cause bubbles coalescence in gas-liquid operations. Fluxes also increase with pore size, as well as the breakthrough pressure of the membrane; a low thickness reduces the resistance offered by the membrane to mass transport whereas, in membrane distillation, increases the amount of heat lost by conduction. Despite the apparent operational complexity, considerable advantages offered by MCs technology make these devices very useful in many applications. Such extremely compact equipments are able to immobilize the interfaces at the membrane pores due to the hydrophobic nature of the membrane itself, and to create a large contact area for promoting an efficient mass transfer. The possibility to vary stream flow rates independently and without occurrence of loading or flooding, the large interfacial area offered to an efficient mass transport, the high modularity and compatibility for an easy scale-up represent additional advantages over traditional unit operations. Disadvantages are mainly related to the presence of an additional mass transport resistance (the membrane itself) and to quite limited range of the operating pressures below the breakthrough threshold. Moreover, up to now, polymeric membrane replacement costs can be considered another disadvantage of MCs. However, new inorganic or hybrid membrane have been recently developing for membrane contactors applications, thus giving a considerable contribution to overcome this inconvenience.
Definitely, Membrane Contactors Technology offers today new opportunities in the design, rationalisation and optimisation of industrial processes. The possibility of integrating these novel membrane operations together with well-assessed traditional membrane units also appears an attractive way to achieve important benefits in the logic of process intensification strategy. The necessity to support conventional pressure driven membrane operations with additional membrane contactors units has recently emerged as essential requisite for approaching the concept of total raw materials utilization, recycle and reuse.
The design of totally innovative membrane integrated processes in water desalination, in the petrochemical industry, in the agro-food and biotechnological processes, might became a reality in the next future, making realistic the objectives of an industrial sustainable growth.
Some references§ Criscuoli, A., Drioli, E. and Moretti, U., 2003, Membrane contactors in the beverage industry for controlling the water gas composition, Annals of NYAS, 984: 1-16.
§ Curcio, E., Criscuoli, A. and Drioli, E., 2001, Membrane Crystallizers, Ind. Eng. Chem. Res., 40: 2679-2684.
§ Curcio, E., Di Profio, G. and Drioli, E., 2003, A new membrane-based crystallization technique: tests on lysozyme, J. Cryst. Growth, 247: 166-176.
§ Di Profio, G., Curcio, E., Cassetta, A., Lamba, D., Drioli, E., 2003, Membrane crystallization of lysozyme: kinetic aspects, J. Cryst. Growth, 257: 359-369.
§ Drioli, E., Curcio, E., Criscuoli, A., Di Profio, G., 2004, Integrated system for recovery of CaCO3, NaCl and MgSO4·7H2O from nanofiltration retentate, J. Mem. Sci., 239: 27-38.
§ Drioli, E., Curcio, E., Di Profio, G., 2005, State of the art and recent progresses in membrane contactors, Chem. Eng. Res. .Des., 83: 223-233.
§ Di Profio, G., Curcio, E., Drioli, E., 2005, Trypsin crystallization by membrane-based techniques, J. Struct. Biol., 150: 41-49.
§ Drioli, E., Di Profio, G., Curcio, E., 2005, Hybrid membrane operations in water desalination and industrial process rationalisation, Water Sci. Tech. 51: 293-304.
§ Di Profio, G., Perrone, G., Curcio, E., Cassetta, A., Lamba, D., Drioli, E., 2005, Preparation of enzyme crystals with tunable morphology in membrane crystallizers, Ind.Eng. Chem. Res. 44: 10005-10012.
§ Drioli, E., Curcio, E., Di Profio, G., Macedonio, F., Criscuoli, A., 2006, Integrating membrane contactors technology and pressure-driven membrane operations for seawater desalination: energy, exergy and costs analysis, Chem. Eng. Res. Des., 84: 209-220.
§ Drioli, E., Criscuoli, A., Curcio, E., Membrane Contactors: Fundamentals, Applications and Potentialities, Elsevier, Amsterdam.
V.N. BURGANOS
Institute of Chemical Engineering and High Temperature Chemical Processes - Foundation for Research and Technology, Hellas, Stadiou street, Platani, 26504 Patras, Greece(vbur/iceht.forth.gr)
Significance of Membrane Structure on Separation Phenomena
The mass transport rate of a species through a membrane is primarily a function of the molecular properties of the species and its interaction with the material that makes up the membrane. The selectivity is, therefore, a strong function of the internal structure properties at the operating conditions. A better understanding of the membrane microstructure and its role in the separation process is important in the development of new and more efficient types of membranes. Moreover, microporous materials like zeolites, are expected to have multifunctional properties thanks to their subnanosized pores, including tailored molecular sieving and selective entrapment of molecules.
Transport in the Interior of Membranes
Transport through membrane materials is a non-equilibrium process, and separation of chemical species results from differences in their transport rates. Membranes are normally classified according to their mean pore size or to the molecular size of the materials that are separated by them. Membranes with pore size of 5000 nm or greater are usually particulate filters. Microfiltration membranes have pore sizes in the range of 100-5000 nm and are capable of removing suspended particles like blood cells and latex emulsions. Ultrafiltration membranes have pore sizes in the range of 2-100 nm and can remove large molecules like albumin or pepsin within this range. Nanofiltration membranes can separate small molecules like divalent salts, dissociated acids, and sugar and have a pore size of less than 1 nm. RO membranes separate material like sodium and chloride on the molecular level and have pore sizes in the range of few angstroms. Gas separation requires membranes with a smaller pore size.[1],[2],[3] In most practical situations, there is a distribution of pore sizes in the membrane, and thus the gas permeability is actually influenced by a combination of transport mechanisms. In practice, when smaller pore sizes are pursued in a preparation process, the membrane porosity is usually reduced as well, thus reducing in turn the gas transport rate through the membrane. This trade off between pore size and porosity is a major subject of membrane research aiming at material development with increased process-scale efficiency.
Membrane Structure Models and Reconstruction Methods
Because of the tremendous significance of the internal structure of membranes on their performance, especially in separation or preferential transport (e.g., fuel cells, membrane reactors) applications, several efforts have been made to develop models and simulators of the membrane structure. Depending on scale, one can roughly divide them into microscopic models and mesoscopic models. In the former case, the nature of the membrane material is obviously of particular importance, as explained below.
The structure of polymeric and inorganic membranes that are used in gas separation can be reconstructed at the atomistic level, in order to allow for a detailed study of the gas molecule interaction with the membrane material. Atomistic simulations of the structure of inorganic membrane materials (e.g., FAU or ZSM zeolites, SiO2) can be realized using Molecular Dynamics and Monte Carlo techniques. The reconstruction of inorganic materials at the molecular scale usually includes the following stages. The atoms of the membrane materials are first positioned at reference bulk lattice sites, taken from literature and/or experimental data. The potential energy of the configuration is calculated using some force field description, which usually involves an analytical expression for the energy of a molecular system in terms of the positions of all its atoms. This is usually called an energy surface. Both intramolecular and non-bonded atomic interactions are taken into account. These data are obtained using either empirical or ab-initio data and are available in databases of academic or commercial software. Depending on the available data and the accuracy needed, simple energy descriptions, like the Universal Force Field (UFF), or more accurate albeit computationally complex force fields, like the Compass FF can be utilized. Boundary conditions are then set, either periodic or of the free surface type. The final step is the total potential energy minimization of the system, using, for instance, the Steepest Descent or Conjugate Gradient techniques, and addition of cation or other bulk/surface defects (where appropriate) in an iterative scheme. Using a force field, several quantities can be computed, such as momenta, interaction energies, conformational energy barriers, free energies, etc. The atomistic structure is the basis for MD and MC calculations to probe the locations, conformations, and motions of molecules, in order to reach useful conclusions concerning the actual supply of sorbates to the active sites of membrane surfaces.
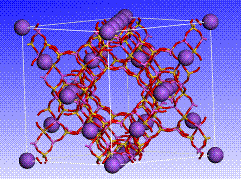
Some of the examples presented here were realized using the Accelrys / Material Studio software and variants of the UFF and Compass FF fields. Sample atomistic reconstructions of equilibrated SiO2 and Na-FAU are shown in Figures 1-2.
 |
|||
 |
|||
| Figure 1. Atomistic reconstruction of SiO2 | Figure 2. FAU structure with equilibrated Na+ cations |
The derivation of macroscopic flow and transport parameters - such as permeability, diffusivity, sorption isotherms, as well as (perm)selectivity - from pore scale properties is still an open problem. In recent studies,[4] promising attempts were made to relate permeability and dispersion to their microscopic origins. In most cases this is done by solving local or average field equations at the pore level of reconstructed porous structures. Other studies consider the behaviour of a packing under the action of various physical forces, but starting from an ordered or random grain distribution. The main approaches to provide a pore space representation are discussed next.
Random packings of hard spheres, discs, and spheroids have been the subject of considerable attention for many years, mainly due to their importance in powder technology and in understanding the structure of amorphous, porous, and random membrane materials. Representation of the structure of porous membranes is attempted by stochastically modelling the outcome of the actual fabrication process (e.g., random assembly or sequential deposition of particles) with or without reference to the detailed physics of the process. Procedures based on Monte Carlo methods are implemented, where each time a number of test grains are inserted, but only selected movements are allowed that lead to minima of position or energy.
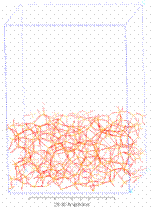
In random sequential deposition of overlapping or non-overlapping particles, the particles are placed in space either randomly, or under the influence of some external force, usually gravity. Ballistic random sphere packs are considered to be realistic representatives of compacted materials[5] (see Figure 3). Procedures based on Monte Carlomethods, similar to the random packing concept, are also available.
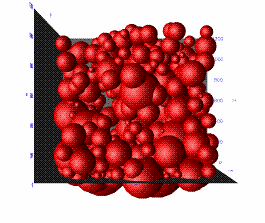 |
 |
| Figure 3. Reconstructed SiO2 by ballistic packing | Figure 4. Reconstructed ceramic membrane |
Pixelized media (fluid mosaic)
The binary representation of porous membranes can provide a direct means for the characterization of their internal structure. Accurate predictions of the Knudsen and intermediate diffusivities in three-dimensional pixelized porous media can be obtained based on molecular trajectory computations. The main advantage of these approaches is that they sidestep resorting to the commonly used concepts of pore, grain, or fiber models, which introduce inevitably a significant degree of approximation to the actual structure.
A classical stochastic reconstruction method is based on the truncation of Gaussian random fields and is capable of generating synthetic pore spaces with specified porosity and autocorrelation function.
Another class of stochastic methods is based on minimizing some group of predefined properties (figure of merit) or "energy", such as porosity or specific area, so as to produce the most reasonable models, which have the lowest values of the figure of merit. This optimization can be achieved by simulated annealing, a procedure for minimizing multidimensional functions. A simulated annealing global optimization method mimics the behavior of a slow cooling solid in a heated bath.
Random walk and Brownian motion have been ubiquitous models in reconstruction of many structures of technological interest.[6] A sample of a reconstructed ceramic membrane structure obtained with the fractional Brownian motion technique is given in Figure 4.
Restricted diffusion and sorption in inorganic membranes
Simulations of the restricted diffusion and sorption of small sorbate molecules can be performed by Molecular Dynamics (MD) and Monte Carlo (MC) methods. Specifically, the motion of the sorbate molecules in the vicinity of the surface layer or in the interior of the material structure is simulated, taking into account the sorbate-sorbate and sorbate-surface interaction forces (see Figure 5). In the MD method, the classical equations of motion are solved numerically with an appropriate integration algorithm. First, an initial configuration and set of velocities are chosen, which are consistent with the desired temperature. Subsequently, the equations of motion are integrated until sufficient equilibration is achieved.[7] Statistical estimates of the system properties are then extracted from the ensemble quantities, including diffusion coefficients, average energies, conformational distributions, and free energies.[8]
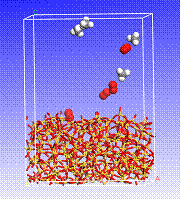
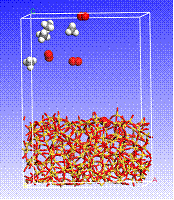
Figure 5. Simulated snapshots of four CH4 and four O2 molecules in the vicinity of a SiO2 surface (view of a three-dimensional, periodic cell).
Among other quantities, the mean residence time (MRT) of the sorbate molecules can also be calculated at the end of a simulation run. MRT is the ratio of the time that the diffusing molecules of a specific type spent in the vicinity of the membrane surface prior to their adsorption, driven by sorbate-sorbate and sorbate-sorbent forces, to the total diffusing time:
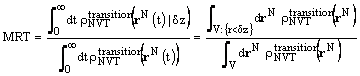
The term 'vicinity' describes the free volume, V, of arbitrary height, dz, over the layer surface. At the end of every simulation time step the existence of each molecule in this region can be monitored. Each existence is considered a "success". MRT is equal to the ratio of the number of "successful" steps to the total number of time steps, for all the molecules of a given type. This property characterizes the transition of molecules from the bulk (free) space into the adsorbing boundary layer, and can be associated to a steady state rate adsorption constant.
The Grand Canonical MC method is usually chosen for sorption simulation and considers constant temperature, pressure, and chemical potential. In this type of simulation, the initial configuration is generated by placing the sorbates at arbitrary positions inside the pore space. Each subsequent configuration is generated by a random translation, rotation, introduction, or destruction of a sorbate molecule and is accepted or rejected using a Metropolis algorithm depending on the configuration energy change. Properties such as the isosteric heat of adsorption and the Henry constant can be extracted.
Separation specific to porous polymeric membranes
Various models have been proposed for the transport of gases in polymers.[9] Among other approaches, molecular mechanisms have been employed to explain gas transport in polymers, invoking the concept of available free volume to provide diffusing channels. The 'hole' or lattice vacancy theory assumes that a certain amount of work must be offered to create or expand a hole in order to accommodate the diffusing molecule. According to the activated complex theory, the diffusing molecule must acquire sufficient energy to overcome the potential energy barrier of the membrane. In the volume fluctuation theory, the diffusing molecule is taking advantage of openings generated by matric fluctuations down a chemical potential gradient.
Molecular dynamics (MD) simulations are increasingly employed to explore the transport of small molecules in polymeric systems. Transition state theory (TST) provides a more approximate treatment of the penetrant diffusion than MD. Attention is focused mainly on the jumps and all the non-jump motions are integrated or ignored. These simulations provide a link between the polymer chain architecture and the penetrant transport that can be employed in the rational design and optimization of separation membranes.
Separation specific to dense polymeric membranes
The mechanism for gas separation by non-porous membranes is different from that by porous membranes. The transport of gases through a dense polymeric membrane is usually described by a solution-diffusion mechanism, according to which the permeants dissolve in the membrane material and then diffuse through the membrane down a concentration gradient. Separation is achieved due to the differences in the amount of material diffusing through the membranes, which, in turn, are driven by differences in the thermodynamic activities at the upstream and downstream faces of the membrane as well as the interactions between the permeate molecules and the membrane material molecules. Recently, both hydrodynamics and nonequilibrium statistical mechanics were used to describe the transport of hydronium ions through the hydrated pores of proton conducting polymeric membranes[10], which play a central role in PEM fuel cells. To this end, considerable effort must be spent to obtain molecular-scale information on the membrane material structure through ab initio electronic-structure calculations.
[1] S. Sourirajan, in Reverse osmosis. New York: Academic, 1970; Sourirajan S, Matsuura T. Reverse osmosis /ultrafilteration process and principle, National Research Council of Canada, Publication No. 241844 1985
[2] R.E. Resting, J. Appl. Polym. Sci. 41, 2739 (1990); R.E. Resting, A.R. Fritzcsche, in Polymeric gas separation membranes, New York: Wiley, 64 (1993).
[3] M. Cheriyan, Ultrafiltration and microfiltration handbook. Lancaster: Technomic, 1998
[4] S.V. Sotirchos and V.N. Burganos, "Transport of gases in porous membranes," MRS Bull. 24(3), 41 (1999).
[5] A. Eidsath, R. G. Carbonell, S. Whitaker, and L. R. Herrmann, "Dispersion in Pulsed Systems .3. Comparison between Theory and Experiments for Packed-Beds," Chem. Eng. Sci. 38(11), 1803 (1983).
[6] E.S. Kikkinides and V.N. Burganos, "Structural and flow properties of binary media generated by fractional Brownian motion models," Phys. Rev. E 59(6), 7185 (1999)
[7] M. Allen and D. Tildesley, Computer Simulation of Liquids. Oxford University Press (1989).
[8] E.D. Skouras, V.N. Burganos, and A.C. Payatakes, "Improved atomistic simulation of diffusion and sorption in metal oxides," J. Chem. Phys. 114(1), 545 (2001).
[9] P. Pandey and R.S. Chauhan, "Membranes for gas separation," Prog. Polym. Sci. 26(6), 853 (2001); D. Hofmann, L. Fritz, J. Ulbrich, C. Schepers, and M. Böhning, "Detailed-atomistic molecular modeling of small molecule diffusion and solution process in polymeric membrane materials", Macromol. Theory Simul., 9, 293 (2000).
[10] S.J. Paddison, R. Paul, and T.A. Zawodzinski, Jr., "A Statistical Mechanical Model of Proton and Water Transport in a Proton Exchange Membrane", J. Electroch. Soc., 147 (2) 617 (2000).
BOHUMIL BERNAUER
Institute of Chemical Technology, Prague, Technicka 5, 166 28 Prague 6
e-mail: bohumil.bernauer/vscht.cz
The significant variety of existing membrane operations is based on relatively simple, compact, and largely clarified fundamental transport mechanisms characterizing mass transfer in the dense or porous membrane layer and at the membrane - fluid interfaces. The understanding and prediction of transport phenomena in the membrane structure is now at least qualitatively possible, also theoretically through the newly available tools provided by molecular simulation, irreversible thermodynamics or relaible phenomenological models.
Prediction of the separation performance of membrane materials requires knowledge on multicomponent diffusion, adsorption parameters and structure parameters. These parameters should be determined by experimental methods and adequate modeling of transport phenomena.
The understanding and modeling of the diffusion process inside the micro, meso or macro pores of membranes poses many challenges for the experimentalist and theoretician alike. The proper description of diffusive transport within membranes is of considerable importance because of the considerable potential importance in chemical technology and new separation processes. A variety of models and techniques have been used to describe diffusion within membranes, e.g. molecular dynamics and Monte Carlo simulation, phenomenological models using Fick's law or Maxwell-Stefan formulation.
In the Wicke-Kallenbach experiments transport in the support layer
can occur by molecular and Knudsen diffusion and by convective
(viscous) flow mechanism. Knudsen diffusion can be neglected in the
support layer if the Knudsen number (Kn) is significantly smaller than
1. Knudsen number is the ratio between the mean free path of the
molecule (![]() ) and the pore diameter (
) and the pore diameter (![]() ):
):
|
|
(1)
(2) |
|
|
|
It is therefore assumed that permeation through the support layer can be described by dusty-gas model to compute concentration field in the support layer. Dusty gas model provides the constitutive flux equations in the following implicit form:
|
|
(3) |
|
|
(4) |
By eliminating the total pressure gradient from equation (3) using equation (4) we obtain the relation between the gradient of partial pressure of i-th compound and the fluxes:
|
|
(5) |
The net flux density Ni through the support are the sum of molecular, Knudsen and viscous flux contributions
|
|
(6) |
METHOD OF SOLUTION
System of ODE with boundary conditions converted to the system of algebraic nonlinear equation by
MODEL PARAMETERS
|
Parameters |
Method |
|
support layer |
Estimated |
|
zeolite and separative layers |
Measured by W-K technique, extrapolated to higher temperature |
|
Adsorption parameters in Langmuir isotherm |
Measured by gravimetric method, extrapolated to higher temperature |
|
Kinetics parameter in reaction rate equation |
Measured in flow catalytic microreactor |
Generalised Maxwell-Stefan form
Transport in microporous crystal is depicted as a sequence of five steps, involving intracrystalline and interfacial processes. According to Barrer [] transport through porous crystal can be described by following consecutive steps:
These are all activated steps, which can be modeled assuming that molecule jumps between low-energy (low-potential) sites. The operating conditions (temperature, concentration of species) and the characteristics of the molecules and the microporous crystalline material determine which of these steps is rate determining. Steps A, B, D, and E are referred to as interfacial processes. Interfacial effects are important for thin membranes and depend on the difference between the activation energy for intracrystalline diffusion. Adsorption on the external surface is usually less strong than adsorption within the crystal, so it is unlikely that steps A or E are to be a rate limiting ones.
The generalized Maxwell-Stefan (GMS) constitutive relations have been successfully applied to many systems to describe diffusive transport phenomena in multicomponent mixtures. The GMS model is based on the momentum transfer between particles in motion. The driving force exerted on any molecule i is balanced by the friction this species experiences with all other species present in the mixture. The essential concept of the theory of diffusion in multicomponent mixture was already available more than a century ago following the pioneering works of James C. Maxwell and Joseph Stefan.
The Generalized Maxwell-Stefan equations provide an adequate basis for the description of multi-component mass transfer in microporous media. The basis of this theory is that the driving force for movement that is acting on a species if balanced by the "friction" with other molecules and vacant adsorption sites that is experienced by that species. We can use the dusty gas model approach to the description of diffusion in adsorbed phase by considering the vacant sites to be the (N+1)th pseudo-species in the adsorbed mixture:
|
|
(8) |
where ![]() ,
the chemical potential gradient of the i-th adsorbed species, is the
driving force exerting on this adsorbed molecule tending to move it the
adsorbed phase. This driving force is balanced by interactions between
i-th and j-th adsorbed molecules and the friction experienced by the
species i from vacant adsorption sites. The qi
represents the surface occupancy of the adsorbed species i and qN+1
represents the fraction of vacant adsorption sites.
,
the chemical potential gradient of the i-th adsorbed species, is the
driving force exerting on this adsorbed molecule tending to move it the
adsorbed phase. This driving force is balanced by interactions between
i-th and j-th adsorbed molecules and the friction experienced by the
species i from vacant adsorption sites. The qi
represents the surface occupancy of the adsorbed species i and qN+1
represents the fraction of vacant adsorption sites.
Illustrative examples of permeation and separation with microporous membranes
Fig. 1 Computed dimensionless transient profile of the n-C4H10
|
|
concepts, materials and applications
J-A. Dalmon
Institut de Recherches sur la Catalyse, CNRS - 2, av. A. Einstein 69626 -Villeurbanne France
Jean-Alain.Dalmon/catalyse.cnrs.fr
Introduction
Catalytic Membrane Reactors (CMRs) are reactors combining in a same unit a catalyst, providing conversion and a membrane that controls transfers to or from the catalyst. In a CMR, the presence of the membrane should improve the performance of the catalyst; in other words, CMRs are not only a sequence of two independent units.
For 20 years, numerous lab-scale studies have been developed to explore the capabilities of membrane and catalyst combinations and regular international meetings gathering specialists of membrane science, catalysis and chemical engineering are organised.
The membrane role, initially acting as a separation barrier, has been extended to other functions, such as reactant contactor. Up to now, most of the applications have considered heterogeneous catalysts and inorganic ceramic membranes. Promising results have been obtained at the laboratory level and few developments scaled up to the pilot level.
Some reviews, books and special issues can provide a complete literature survey of membrane catalysis [1-3].
In what follows, the various types of CMRs will be first described. In a second section, materials (both membrane and catalyst) used in CMR will be introduced. The third part will briefly present examples from our group for each CMR type. They will be detailed, together with other results from the literature, during the oral presentation.
A classification of membrane reactors
There are numerous ways of combining a catalyst and a membrane. Moreover, depending on the membrane role, the catalyst may be placed in a very different situation. All this makes a global analysis almost impossible. A classification of CMRs has been proposed according to the way of combining catalyst and membrane [2]. The main criterion is based on the presence in the CMR of a catalytic membrane (the same material acts as catalyst and membrane) or of an association of a conventional catalyst (packed or fluidized bed) and a membrane that just rules transfers.
Another classification is based on the membrane role [4]. As a matter of fact, as schematized in figure 1, the membrane can have 3 very different functions according to the CMR type:
(i) The membrane can be used to remove a reaction product from the reaction zone. This type of CMR, called extractor, is certainly the most studied. An extractor can be used to increase reaction yields. This is obtained either by improving the conversion in equilibrium-restricted reactions or, in consecutive reactions, by improving the selectivity towards a primary product via its selective extraction through the membrane.
In extractor CMR applications, the membrane permeation should be highly selective towards one product to get a performance improvement: any loss of another species involved in the reaction will be highly detrimental.
(ii) The membrane can control the introduction of one of the reactants in the reaction zone. This type of CMR, called distributor, is used to spread a reactant all along the catalytic zone, in which the other reactant is introduced as usually. In this way, when compared to conventional reactors, though the same (or even larger) amounts of the distributed reactant can be introduced, its concentration is kept at a low level in the entire reaction zone. This low concentration may increase the selectivity of reactions when the distributed reactant can undergo successive additions. Selective oxidations (or hydrogenations) can be improved in
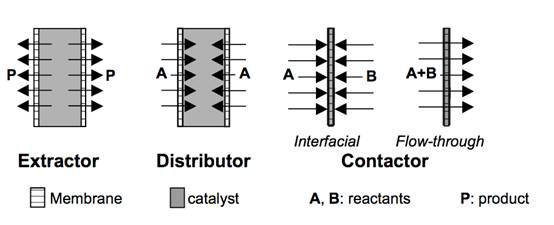
Figure 1. Sketch of the different CMRs
distributor-type CMR if a membrane distributes oxygen (hydrogen). Another advantage of such reactor is related to flammable mixtures. Due to the low O2 concentration in the catalyst bed, the local reactants composition can be kept outside the flammability region, though the total amount of reactants introduced corresponds to a ratio that is forbidden in conventional reactors.
In distributor-type CMR, the membrane acts as a diffusion barrier for one reactant. It should avoid back permeation of other species. More than the membrane pore characteristics, the operative conditions are crucial to control the transfers through the membrane.
(iii) A membrane may be use to facilitate the contact between reactants and catalyst. This CMR type, called contactor, takes advantage of the very unique configuration of the membrane pore, which contrary to pores of conventional solids, presents two distinct ways of access, corresponding to the two sides of the membrane. In contactors, the membrane generally also acts as catalyst support (or is intrinsically active). A contactor can be used following two modes (figure 1). In the interfacial contactor mode, the reactants are separately introduced from each side of the membrane, and meet in the catalyst zone. Such configuration has been used in the case of non-miscible reactants, as in gas-liquid catalytic reactions. It has been pointed out that, contrary to what is generally observed in conventional reactors, the gaseous reactant is no more limiting. Dense polymeric membranes containing metal catalyst encaged in zeolites have also been used as interfacial contactors in the case of non-miscible aqueous and organic reactants. In this case, the polymeric membrane favours the transfer of the organic reactant to the active phase, which allows the contactor to perform better than conventional reactors. The other contactor mode is the flow-through contactor. In this second mode, the mixture of reactants is forced through the membrane, i.e. through the catalytic pores. Contact time and permeation regime in the active pore itself can be directly adjusted from the operative conditions and adapted to required values, which is hardly feasible in conventional reactors. Such reactors have been used for gas and gas-liquid reactions, and showed improvements in activity and selectivity.
CMR Materials
Membranes
Most of the first CMR studies were based on catalytic reactions requiring temperatures above those that polymeric membranes could withstand. This led to focus on inorganic membranes. Both dense and porous inorganic materials have been used. The membrane choice highly depends on the CMR type and application.
Dense inorganic membranes.
As dehydrogenation is one of frequently studied reactions, dense palladium membranes, selective for hydrogen permeation, have been used in extractor-CMR to improve the reaction yield by selective H2 removal from the reactor. Most of these membranes are obtained by electroless plating techniques. The film is formed on top of a porous ceramic or metallic support. Pd is often combined with Ag to improve the resistance of the material at low temperature.
Beside metals, dense oxides have been also considered for membrane production, mainly for selective oxygen permeation in oxidation reactions. Such materials received an important support via several international consortia gathering leading industrial partners. Mixed conducting (ionic and electronic) oxide membranes, such as perovskites, were prepared using salts as precursors, by calcination at high temperature and sintering the powders under high pressures. Selective O2 permetion occurs at high temperature (800°C). The main application was the separation of oxygen from air. The O2 production is an economical key step in the syngas production from methane partial oxidation. As this reaction also occurs at high temperature membrane and catalyst are associated in a same unit. This is not stricto sensu a CMR, though it is has been proposed that the catalyst can take benefit of the presence of highly reactive O species at the membrane surface and that the absence of gaseous oxygen limits deep CH4 oxidation and flammability problems.
Porous inorganic membranes.
If dense membranes are highly selective, they often suffer from low permeances and problems for large-scale production. Porous membranes are, in principle, less selective, but generally provide enough permeating fluxes to be combined with catalytic performances.
Depending on the application, microporous or mesoporous layers have been used. When high separation selectivity is required (extractor CMRs), microporous membranes may compete with dense membranes. Such microporous membranes can be obtained either by sol-gel techniques (as mesoporous membranes) or using zeolite application on a macroporous ceramic (or metallic) support. Microporous membranes can be used as molecular sieving systems, blocking permeation of species whose diameter is larger than the pore size. However, especially in the case of zeolite membranes, adsorption properties very often rule the permeation selectivity. In a mixture made of a small-size non-adsorbing molecule with a larger adsorbing species, the zeolite membrane separation can be in favour of the larger molecule that occupies all the microporous volume, thus blocking permeation of the smaller species.
Mesoporous inorganic membranes generally do not provide enough separation selectivity (based on Knudsen diffusion) for gas-phase catalytic reactions. They are mainly used in distributor or contactor CMR modes where separation selectivity is generally not the leading membrane effect.
Polymeric membranes
As above-mentioned, examples of CMRs using organic membranes are less numerous than those using inorganic materials. However, in principle, all the modes represented in Fig. 1 can be implemented with polymeric membranes. Extractor and contactor CMRs have been considered in low temperature catalytic reactions.
Catalysts
When compared to membranes, catalysts for CMR received much less attention. However, the specific conditions prevailing in membrane reactors may modify the catalyst state and properties in comparison to those in conventional systems [5]. In some cases, adapted catalysts have to be designed to get higher performance in CMRs.
CMR case studies
Extractor
Isobutanedehydrogenation [6]
The isobutane dehydrogenation was studied in two extractor-type CMRs. Combinations of a Pt-based catalyst with two different membranes, a Pd membrane obtained by electroless plating and a MFI zeolite membrane obtained by hydrothermal synthesis, were compared. Both CMRs give better results than conventional reactors. However, though the two membranes presented different separative properties, the two CMRs showed very similar yields in the isobutane dehydrogenation. This has been attributed to the limitation of both CMR performance by the catalyst lack of efficiency. A modeling approach, that combines catalysis kinetic law for and membrane gas transfer equations also contributes to the description of the CMRs performance.
Xylene isomerization [7]
A zeolite/alumina membrane was used to successfully separate xylene isomers. It was then applied, as a selective membrane, in an extractor type catalytic membrane reactor (CMR), used to enhance the xylene isomerization reaction selectivity towards para-xylene. The results of the CMR in different configurations (permeate-only and combined permeate-and-retentate mode) were compared to conventional fixed-bed reactor results. In both cases, the selectivity was significantly enhanced (up to 100% in permeate-only mode). In the combined mode, the CMR also provided a net increase in productivity over the conventional reactor.
Distributor
n-butane selective oxidation [8]
The n-butane selective oxidation has been studied in a membrane reactor, using high butane concentrations. Thanks to the oxygen distribution by the membrane, it is possible to keep the local composition outside the flammability zone. A MFI ceramic membrane was used to distribute oxygen (or part of it) in the catalyst bed, made of a VPO mixed oxide, either conventional or Co-doped. In a first step, the effect of the oxygen distribution has been studied, showing that, under standard reactant mixtures (O2/butane=12, low butane concentration), the membrane reactor performed very close to the conventional one. Under high butane concentrations, the VPO system suffered from a drastic decrease of the selectivity towards maleic anhydride (MA). The addition of cobalt to the VPO catalyst allowed keeping the MA selectivity at a high level (75%). The combination of the CoVPO catalyst and the MFI membrane was used to explore the membrane reactor performance with high butane concentrations in the feed, corresponding to the flammability zone in a conventional reactor. For these conditions, the MA productivity was 3 times higher than that observed with the conventional reactor.
Contactor
Wet Air Oxidation process [9]
A new and innovative method for oxidation of dissolved compounds in water - the "Watercatox" process - has been developed in order to reduce the chemical oxygen demand and the total organic carbon in industrial wastewaters. This process is the result of a European Fifth Framework Program project. It can operate at much lower temperatures and pressures than conventional wet air oxidation or incineration, and it offers much smaller volume requirements than biological treatment plants. The operating principle of the Watercatox process is the oxidation of the dissolved molecules using oxygen from air within a catalytic membrane reactor in an interfacial contactor configuration. The catalytic contactor membranes, as well as the operating conditions, have been up-scaled from lab-scale to pilot unit. The technological efficiency was demonstrated by the results obtained using the pilot test unit on different industrial effluents from several origins.
Conclusion
Thanks to a large research effort at a laboratory level, important progresses have been made in CMRs on the basis of various catalyst/membrane combinations. Applications cover a large domain, from petrochemistry to fine chemicals or environmental issue remediation. However, in some cases, membrane costs could be a limiting factor and long duration experiments are still needed.
Though CMRs presented here are, for the time being, very far to reach the industrial development of membrane bioreactors, first CMR pilots have been recently developed.
References
[1] J-A. Dalmon, "Handbook of Heterogeneous Catalysis", VCH Publ. (1997), 1387
[2] J. Sanchez, T.T. Tsotsis "Catalytic Membranes & Catalytic Membrane Reactors", Wiley (2002).
[3] Catalysis Today, special issues on membrane catalysis, 25,3-4 (1995); 56,1-3 (2000); 67,1-3 (2001); 82,1-4 (2003); 104,2-4 (2005)
[4] S. Mota, S. Miachon, J.-C. Volta, and J-A. Dalmon, Catalysis Today, 67, (2001), 169.
[5] S. Miachon and J-A. Dalmon, Topics in Catalysis, 29 1-2 (2004) 59
[6] L. van Dyk, S. Miachon, L. Lorenzen, M. Torres, K. Fiaty, J-A. Dalmon, Cat. Tod, 82, (1-4) (2003), 167.
[7] L. van Dyk, L. Lorenzen, S. Miachon, J-A. Dalmon, Cat. Today, 104(2-4) (2005), 274.
[8] A. Cruz-López, N. Guilhaume, S. Miachon and J-A. Dalmon, Catalysis Today, 107-108, 949.
[9] E. E. Iojoiu, J. C. Walmsley, H. Raeder, S. Miachon, J-A. Dalmon, Cat. Tod., 104(2-4) (2005), 329.
G. JONSSON
Department of Chemical Engineering, Technical University of Denmark, Building 229 DTU, DK-2800 Kgs. Lyngby, Denmark, (gj/kt.dtu.dk)
Introduction
Fermentation processes are often inhibited by either products or biproducts produced by the microorganisms thereby limiting the maximum product concentration which can be obtained during the fermentation. Also it will influence the maximum biomass concentration which can be obtained thereby decreasing the production rate. A typical batch fermentation as sketched in Fig. 1 consist of a lag phase where the microorganisms "wake up" followed by an exponential growth phase which goes into a stationary phase depending on the inhibiting effect of the different products. Removing these products directly from the fermentation by a membrane separation process makes it possible to operate at much higher biomass concentrations thereby increasing the production rate as well as the final product concentration which might have a beneficial influence on the further downstream processing. Depending on the type of products or inhibiting biproducts such membrane processes can be either ultra/microfiltration (size related separations), membrane distillation (volatile products like bioethanol) or electro membrane processes (charged products like organic acids or bases). Combining fermentation directly with membrane separation will further make it possible to operate a high cell density in even a continuous fermentation process which is today used on large scale in waste water treatment plants using membrane bioreactors (MBR) where the membrane plant can either be internal (submerged membranes) or external (recycle loop) to the biological waste water treatment plant.
Figure 1. Biomass growth rate in a typical batch fermentation.
The lecture will discuss the different advantages and problems related to using each of the three membrane separation processes: 1) ultra/microfiltration, 2) membrane distillation and 3) electro membrane processes directly coupled to fermentation processes. The main focus will however be on a newly developed electro membrane process named reverse electro-enhanced dialysis (REED):
REED Technology
The Reverse Electro-Enhanced Dialysis (REED) setup and design combines elements from electrodialysis reversal (EDR) and Donnan dialysis (DD) operations.
The REED unit improves fermenter productivity by continuously removing inhibiting organic acids and replacing them by alkaline hydroxide ions. This removes the acids, which would otherwise inhibit the growth of the microorganisms, as well as provides a pH regulation of the fermenter. Fermentation broth is continuously taken from the fermenter and pumped into the REED unit and then back to the fermenter.
The REED unit is a plate-and-frame module as demonstrated below. Ion-exchange membranes are placed in layers, separated by flow spacers, which facilitate the passage of the fermentation broth between each set of membranes. Inside the REED unit, the broth is diverted into every second flow spacer, where it streams along the surfaces of the two ion-exchange membranes on either side of the spacer. Inside the alternating flow spacers, an alkaline stream (the Dialysate) flows without direct contact to the broth. The ion-exchange membranes are positively charged, which allows the negatively charged ions to be exchanged through the membranes. Positively charged ions are excluded from passing through the membranes because they hold similar charges.
Figure 2. Set-up of the REED module.
The ion transport is carried by electrical current, which is added across the module from electrodes placed at each end of the stack. Since the concentration of organic acids is significantly higher than that of hydroxide ions in the broth, the current is mainly carried by the organics acids, which are transported to and through the ion-exchange membranes. In the alkaline solution, the concentration of alkaline hydroxide ions is much higher than that of the organic acids, which enters the solution through the membranes. Thus, in the alkaline solution the electrical current is mainly carried by hydroxide ions moving through the ion-exchange membranes and into the fermentation broth. This overall exchange of negative ions preserves electro-neutrality.
Bio-matter in the form of microorganisms, yeast extracts and other organic particular components as well as proteins and other high-molecular components are too bulky to pass through the dense ion-exchange membranes. But the bio-matter and proteins tend to foul the membrane surfaces, causing a build-up of organic matter on the membranes inside the flow spacers with fermentation broth. This membrane fouling causes a steady increase in the electrical resistance of the membrane stack. All the negative ions migrate in the same direction, towards the positive electrode (anode). Inside each flow spacer carrying fermentation broth, organic acids are leaving to one side while hydroxide ions are entering from the opposite site, as demonstrated in the process setup sketch above. The hydroxide ions entering the flow spacer through the ion exchange membranes destabilize any build-up of fouling on that side, which they enter. On the other side of the flow spacer, fouling builds up, only reduced by the shear stress from the bulk flow in the spacer along the membrane surface, which drags some of the top fouling layer off. The symmetrical setup of the REED system allows the direction of the electrical current to be completely reversed while the overall separation is continued. The principle is sketched below.
Figure 3. Sketch of the effect of the current reversal on membrane fouling.
In figure 3A, the negative ions migrate towards the positive electrode (anode), meaning that hydroxide ions enters the flow spacers with fermentation broth from the left, and fouling builds up on the right membrane surface. When the direction of the electrical current is reversed (figure 3B), hydroxide ions enter the flow spacer from the right, destabilizing and removing the fouling layer, while a new fouling layer builds up on the left membrane surface. The current is reversed at regular intervals of 5-10 minutes. The interval is determined by the build-up of fouling; when the build-up is severe, short periods are necessary. Each reversal reduces the overall separation efficiency due to the change in concentration gradients inside the membranes and the intervals should be kept as long as possible. The alkaline solution, which collects the organic acids in the form of acid salts (e.g. Sodium Lactate) is either discarded or recycled through the REED unit until sufficient amounts of lactate is collected in the solution. By continuously adding fresh alkaline solution to the REED system, the efficiency is high, but the amount of alkaline solution which must be discarded, is also high. Improving the utilization of the alkaline solution reduces the process efficiency and demands higher energy consumption and membrane area, so careful evaluation of these parameters must be taken into account when considering the system design.
Case study
The ability of the reverse electro-enhanced dialysis (REED) separation system to extract growth inhibiting lactic acid during live lactic acid bacteria fermentations has proven significant boosts in productivity and product yield for a Gram-positive expression system using Lactococcus lactis as a host organism for production of recombinant proteins for use in humans [1].
Lc. lactisproduces lactic acid from sugars as integral part of its primary energy metabolism, and if the cells are provided with sufficient glucose, growth as well as protein production will be limited by accumulation of lactic acid in the fermentation broth. Inhibition of growth and protein production can be delayed, but not prevented by titration of the lactic acid with base. Lactic acid and lactate also serve to induce production with the P170 system promoter; again the inducing concentration will be higher if pH is kept high with base addition. At a given pH value, the optimal inducing level of lactate will be severely but not absolutely growth inhibiting.
The patented electro-membrane separation process, REED, provides a technology for removing lactate from fermentation broth, while retaining cells, proteins, sugars and most other feed components. When the process is controlled to keep a constant pH in the fermenter, the amount of lactate in the broth will also be kept constant. This means that the level of growth inhibition and of expression system induction can be controlled during fermentation.
The same principles used for the P170 expression system in this abstract can be transferred to other fermentation and expression systems whether mammalian or bacterial.
REED fermentations
For REED fermentations, a fed-batch fermenter is fitted with an external loop, which passes through a membrane stack (Figure 4), where lactate is selectively extracted, before the acid-depleted ferment is recirculated to the fermenter.
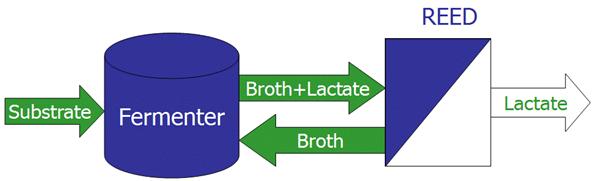
Figure 4. REED fed-batch fermentation setup.
The fed-batch fermentation is operated in three steps. First, the fermentation runs through its lag phase and enters the exponential growth phase. When a certain non-inhibiting concentration of lactate is reached, the REED system takes over pH-control of the fermenter by continuously extracting newly produced lactate, replacing lactate ions with hydroxide ions, and thus maintaining constant fermenter pH. Through this method, it is possible to obtain significantly higher biomass owing to the uninhibited growth compared to standard fermentations. A concentrated solution of fresh substrate and other vital growth components are fed to the fermenter to supplement the growth.
When sufficient biomass has been produced, the fermenter's lactate concentration is increased through addition of sodium lactate to reach a new level, which triggers the expression system, but still remains below the inhibition level for lactate. At this third fermentation phase, the increased biomass expresses the pharmaceutical protein at a significantly higher rate compared to what can be obtained in similar batch fermentations. Since freshly produced lactate is continuously removed to maintain the desired lactate level in the fermenter, the production phase can be prolonged significantly resulting in yield improvement.
Results
Laboratory experiments were successfully carried out for different strains with similar results. When a standard batch would normally stop production due to inhibition, the cells in the REED fed-batch fermentation continued to grow and express the protein. Figure 5 shows the difference in biomass (OD600) and produced recombinant protein between standard batch production and REED fed-batch fermentation. The time of induction, where lactate is added to the fermenter to promote protein expression is shown in figure 5.
Figure 5. Comparison between biomass output (OD) and produced protein for REED fed-batch fermentation versus standard batch fermentation.
Conclusion
The results show that the REED process is able to boost both productivity and product yield dramatically for the P170 expression system, and makes it feasible to produce even normally low-yield pharmaceutical components.
[1] L. Bredmose, S.M. Madsen, A. Vrang, P. Ravn, M.G. Johnson, J. Glenting, J. Arnau and H. Israelsen, "Development of a Heterologous Gene Expression System for use in Lactococcus lactis", O.-W. Merten, et al. (eds.), "Recombinant Protein Production with Prokaryotic and Eukaryotic Cells", p. 269-275, Kluwer Academic Publishers (Netherlands) 2001.
[2] A. Garde, Ph.D. Thesis: Production of lactic acid from renewable resources using electrodialysis for product recovery, 2002, ISBN 87-90142-84-5
SUZANA PEREIRA NUNES
GKSS Research Centre, Max-Planck-Str. 1, 21502 Geesthacht, Germany, e-mail nunes/gkss.de
The need for reducing the worldwide CO2 emission and searching for independence of fossil fuels has increased the motivation to establish fuel cell and hydrogen technology in all sectors of energy. In this task there are important challenges for membranes. Examples are:
- H2 separation in petrochemical streams
- CO2 sequestration
- Membrane reactors involving H2
- Fuel cells, electrolysers
![]()
 Hydrogen
is still being produced in large scale by the petrochemical industry.
Although the hydrogen technology is expected to be very important in
the future, the world will still be using fossil energy in the next
decades. It is decisive to make all the processes involving fossil fuel
as clean as possible. Here membranes can play a decisive role in the
hydrogen separation and processing and CO2 sequestration.
Many of the tasks for hydrogen separation in the petrochemical industry
take place at quite high temperature, making inorganic membranes in
these cases the only choice. Among the most investigated inorganic
membranes for hydrogen separation are silica membranes based on
molecular sieve mechanism and palladium membranes based on the strong
interaction of hydrogen and palladium, which makes them extremely
selective. More recent a new class of mixed electron and proton
conductive oxides has emerged allowing a very selective of hydrogen
through the membranes. However for the temperature range below 250oC
inorganic membranes can hardly compete with polymer membranes, which
are cheaper and easy to manufacture in large scale. Asymmetric
polymeric membranes (Figure 1) for hydrogen separation based on Matrimid®,
a commercial polyimide, have been developed for manufactured in
technical machines at GKSS. The H2/CH4
selectivity is ca. 100 and CO2/CH4 selectivity 39
with gas permeability of 1 m3(STP)/m2 h bar [1].
Hydrogen
is still being produced in large scale by the petrochemical industry.
Although the hydrogen technology is expected to be very important in
the future, the world will still be using fossil energy in the next
decades. It is decisive to make all the processes involving fossil fuel
as clean as possible. Here membranes can play a decisive role in the
hydrogen separation and processing and CO2 sequestration.
Many of the tasks for hydrogen separation in the petrochemical industry
take place at quite high temperature, making inorganic membranes in
these cases the only choice. Among the most investigated inorganic
membranes for hydrogen separation are silica membranes based on
molecular sieve mechanism and palladium membranes based on the strong
interaction of hydrogen and palladium, which makes them extremely
selective. More recent a new class of mixed electron and proton
conductive oxides has emerged allowing a very selective of hydrogen
through the membranes. However for the temperature range below 250oC
inorganic membranes can hardly compete with polymer membranes, which
are cheaper and easy to manufacture in large scale. Asymmetric
polymeric membranes (Figure 1) for hydrogen separation based on Matrimid®,
a commercial polyimide, have been developed for manufactured in
technical machines at GKSS. The H2/CH4
selectivity is ca. 100 and CO2/CH4 selectivity 39
with gas permeability of 1 m3(STP)/m2 h bar [1].
Membranes for fuel cell
![]()
 In
the low temperature fuel cell a polyelectrolyte membrane is a core
component to transport protons from the anode to the cathode generating
electricity (Figure 2). The requirements for a good membrane are: high
chemical stability, high proton conductivity, low fuel and oxygen
permeabilities, high electronic resistance and preferentially low cost.
The membrane surfaces are in direct contact to a finely dispersed
catalyst mainly on Pt base and the electrodes. The main commercial
membrane for fuel cells is based on NafionÒ, a fluorinated
polymer with sulfonated side chains. However well known drawbacks are
the high costs, and mainly two technical aspects:
In
the low temperature fuel cell a polyelectrolyte membrane is a core
component to transport protons from the anode to the cathode generating
electricity (Figure 2). The requirements for a good membrane are: high
chemical stability, high proton conductivity, low fuel and oxygen
permeabilities, high electronic resistance and preferentially low cost.
The membrane surfaces are in direct contact to a finely dispersed
catalyst mainly on Pt base and the electrodes. The main commercial
membrane for fuel cells is based on NafionÒ, a fluorinated
polymer with sulfonated side chains. However well known drawbacks are
the high costs, and mainly two technical aspects:
(1) for portable application, when using methanol as primary fuel, NafionÒ is too permeable, allowing methanol to migrate to the cathode and decreasing the cell performance;
(2) for automotive application, for which the preferential operation is considered to be above 100oC and at low humidity, the membrane looses conductivity.
To overcome these problems many alternative polymeric materials are under investigation. Most of them are sulfonated polymers. The preparation varies from post sulfonation of commercial polymers to grafting of sulfonic segments in a polymer membrane by gamma [3] or electron [4] radiation and the polymerization from sulfonated monomers. Fluorinated polymers are usually more chemically stable, however more expensive and more difficult to synthesize. They have been reviewed recently [5].
A large number of non-fluorinated polymers are now under investigation. Some of these activities have been reviewed in recent publications [2, 6]. Approaches include the sulfonation of commercial polymers and the polymerization of new functionalized polymers. One of the first polymers chosen for sulfonation was polysulfone. More recently the synthesis of stable polysulfones from sulfonated monomers has been explored.
The investigation of different variants of sulfonated polyetherketones has been widely described in the literature: (polyetherketone, poly(ether ether ketone) [7] , poly(ether ketone ketone) [8] and poly(ether ether ketone ketone) (sPEEKK) [9], poly(oxa-pphenylene-3,3-phtalido-p-phenylene-oxa-p-phenileneoxy-phenylene) (PEEK-WC) [10]. The comparison between different structures of polyetherketones prepared by polycondensation of sulfonated monomers has been recently reported [11].
Polyphosphazene sulfonic acids [12] offer a unique combination of inorganic backbone with high stability, high ionic density and structural diversity with cross-linking alternatives. Polyphosphazenes with sulphonamide functionalisation have been reported with high proton conductivity [13]
A big challenge for fuel cell membranes is a good performance at temperatures higher than 100oC. Reviews on materials under investigation to overcome this problem have been published by Alberti and Casciola [14], Q. Li et al. [15] and Hogarth et al. [16].
Polybenzimidazole (PBI) is one of the few polymers under consideration for high temperature operation. For that the membrane was immersed in concentrated phosphoric acid to reach the needed proton conductivity. Operation up to 200oC is reported [17]. A disadvantage of this class of membranes is the acid leaching out during operation, particularly problematic for cells directly fed with liquid fuels. Additionally the phosphoric acid may adsorb on platinum surface.
The combination of organic and inorganic materials to develop membranes for fuel cell has become a versatile approach. Early reports and patent applications of Stonehart and Watanabe [18] and Antonucci and Arico [19] claim the advantage of the introduction of small amounts of silica particles to Nafion® to increase the retention of water and improve the membrane performance above 100oC. The effect is believed to be a result of the water adsorption on the oxide surface. As a consequence the back-diffusion of the cathode-produced water is enhanced and the water electro-osmotic drag from anode to cathode is reduced.
Another important motivation for the development of organic-inorganic membranes for fuel cell is the reduction of methanol and water permeability, which are highly relevant aspects for the establishment of the DMFC technology. The inorganic phase can be included in different ways, starting from the simple dispersion of isotropic particles (SiO2, ZrO2, etc), introduction of fillers with high aspect like layered silicates and the in situ generation of an oxide phase in the polymeric matrix by dispersion of inorganic precursors followed by their hydrolysis and polycondensation [20, 21]. The reduction of methanol and water permeability of sulfonated poly (ether ether ketone membranes) with the generation of ZrO2 and SiO2 in the membrane casting solution was reported in different papers [22]. These fillers are isotropic. Inorganic fillers like layered silicates, with high aspect ratios, are expected to lead to a more effective permeability reduction in membranes.
Silica and silicates are usually rather passive fillers. Active fillers able to contribute also for the proton conductivity include zirconium [14, 23] and boron phosphates [24] and heteropolyacids [25].
References
K. BOUZEK, S. MORAVCOVÁ, M. PAIDAR
Department of Inorganic Technology, Institute of Chemical Technology in Prague, Technická 5, CZ‑166 28 Prague 6, Czech Republic (bouzekk/vscht.cz, http://www.vscht.cz)
The ion selective membranes represent modern materials with a broad range of applications based on theirs specific properties. The ion selective membrane is typically applied as a so‑called solid polymer electrolyte. In this role membrane may fulfil two tasks. The more classical one consists in the separation of the anode and cathode compartments avoiding thus mixing of the electrolytes. This is either to hinder degradation of the electrochemical synthesis product on the counter electrode or to allow selective transport of the ions from the individual electrolyte streams. A typical example of such type of large‑scale industrial application represents the brine electrolysis to produce chlorine and caustic. In some applications the membrane replaces liquid electrolyte almost completely. Typical example of this arrangement represent ion selective electrodes, acidic water electrolysis and during the few last decades also PEM (proton exchange membrane) type fuel cells. There are more reasons for the replacement of the liquid electrolyte by the polymer membrane. In the case of the ion selective electrode it is typically selectivity of the membrane to particular ion or group of ions. In the electrolytic cells and fuel cells the reason consists in a substantial simplification of the cell construction and minimisation of danger of the electrolyte leakage and corrosion of the cell construction parts. At the same time reduction of the ohmic drop may be attained. This is due to the extremely low thickness of the modern ion selective membranes and theirs high conductivity.
The role of the membrane in the electrochemical systems is identical to those of the classical electrolyte. It means the electrical insulation of the electrodes and at the same time sufficiently conductive ionic contact between them allowing to close the cell electrical circuit with minimal ohmic potential loss. The demands on the membrane correspond to its task in the system. It is obvious, that in the electrochemical system sufficient ionic conductivity and mechanical and chemical stability represent the primary requirements. Beside them, various additional parameters have to be fulfilled according to the particular application, e.g. permeability and/or selectivity. Additional parameters are determined in order to allow more detail characterisation and understanding of the membrane behaviour. Following main representatives of the last group may be introduced: ion exchange capacity, ion exchange kinetics and degree of swelling. Since the aim of this contribution is to discuss in more detail electrochemical characterisation of the membrane materials with focus on theirs utilisation in the fuel cell technology, we skip majority of the parameters mentioned and focus just on the few of them relevant to this assignment.
The ionic conductivity of the membrane is one of the parameters being determined for each ion selective membrane prepared. This was already in history one of the main characteristics and it remains true until the recent days. Just the instrumentation and techniques have developed during the last decades rapidly. The methods used recently can be divided into the two main groups according to the electrical current signal used: direct current techniques and alternating current techniques.
Direct current techniques represent the original approach, which has been used historically and which is up to now well established especially in the industrial laboratories. The main advantage of this approach consists in the similarity to the membrane industrial application. It is based on the load of the membrane by the defined current density and determining the ohmic potential drop. Considering, that the membrane resistance can be approximated by the linear electrical resistance element, its value can be simply evaluated from these data using the known membrane thickness. The main danger of this approach consists in the polarisation of the membrane‑solution interface. Polarisation by the direct current causes ionic flux across the membrane. It results in the depletion of the boundary layer on one side and enrichment on the opposite one. This has two consequences. Increase in the concentration difference on the membrane results in a building up the liquid junction potential. This phenomenon represents an important artefact bringing uncertainty into the measurement of the ohmic potential drop. If the current density is to high or the electrolyte concentration to low, the concentration of the electroactive specie at the interface may fall down to zero. In such a case the potential drop on the interface between the membrane and electrolyte solution increases rapidly in order to provide sufficient amount of ions. Theirs source represents in the classical systems the water decomposition. Potential drop higher than 1.2 V on the membrane‑solution interface is necessary to decompose this molecule.
|
|
|
|
|||
|
FIG. 1: Schematic sketches of the cells for the membrane conductivity measurements. A - two electrodes arrangement for the electrolyte solution environment, B - four electrode arrangement for the electrolyte solution environment, C - four electrode arrangement for the gaseous environment. |
|||||
Alternating current techniques have an important advantage over the direct current ones. The periodic alternation of the current load results in keeping almost constant conditions on the both membrane surfaces. Thus, effect of the liquid junction potential and surface polarisation is eliminated. Application of the alternating current implicates appearance of the impedance, i.e. complex resistance, instead of the linear electrical resistivity. It is necessary to keep this fact on the mind when evaluating the data quantitatively. The most accurate method is based on the determining impedance spectra in the sufficiently broad perturbation signal frequency range. From such spectra it is possible by proper mathematical treatment to separate sample resistivity unencumbered with serious experimental errors. In principle two different electrical circuits can be used. The first, simpler one uses two electrodes arrangement. The electrodes used to impose perturbation signal on the system act at the same time as a sensing electrodes. Electrode reaction kinetics is thus included in the impedance spectra obtained. The alternative four electrodes arrangement uses sensing electrode separated from the electrodes imposing the perturbation signal. They are thus free of any electrochemical polarisation processes. Another problem represents the elimination of the electrolyte solution resistance. Electrolyte resistivity is similar to the membrane and it is thus difficult to distinguish between theirs contributions. The instrumental arrangement has to be used, which allows to eliminate into the necessary extend influence of these negative aspects.
The typical experimental arrangements used for membrane conductivity measurements are summarised in Fig. 1. Whereas Fig. 1A shows typical two electrode arrangement used for the measurements in the electrolyte solution, Figs. 1B and 1C summarize the four electrode arrangement cells. First of them is used for the electrolyte solution environment, whereas construction of the second one allows to work in the gaseous atmosphere.
We have to keep on the mind the fact that PEM type fuel cell works in the gaseous atmosphere and thus relevant conductivity experiments have to be performed at similar conditions. In an optimal case membrane sensitivity to its degree of swelling should be investigated and understood. This is not possible in majority of the two electrodes arrangements, one exception being application of the mercury electrodes. This arrangement is, in principle, identical to those shown in Fig. 1A. In this particular case the membrane squeezed between two electrode compartments is equilibrated with a gaseous atmosphere of defined relative humidity. Afterwards compartments are filled by the mercury. Mercury ensures membrane sample to keep its degree of swelling and at the same time precise contact of the electrode with the surface of the membrane. Since mercury is used, hydrogen and oxygen evolution reaction is strongly hindered. When the experiment is performed under the open circuit potential, spectra equivalent to the two electric capacities corresponding to the mercury - membrane interfaces with an electrical resistivity corresponding to the membrane resistivity is obtained. Resistivity is connected in series with the capacities and it is located between them. In the case of the four electrode arrangement the situation is easier, because sample can be exposed to the required gaseous atmosphere, i.e. conductivity at the required degree of swelling can be determined. Since no polarisation take place on the sensing electrodes and since no reaction occurs at these electrodes, theirs impedance spectra correspond to the simple resistivity and as such are they evaluated.
Another highly important parameter characterising the ion selective materials represents ion exchange capacity. This parameter provides information on the concentration of the dissociated functional groups available. It is extremely important information not only from the point of view of the ion exchange processes, but also for the transport characteristics of the ion selective membrane. Whereas on one hand the increase of the ion exchange capacity provides increase in the charge carriers concentration inside the membrane, it causes at the same time increase in the volume of the solvatation water in the membrane interior and thus an increase in its void volume. Increase in the void volume is connected with the two phenomenons. The first one is represented by an increase in the free membrane cross‑section. It allows faster ions movement. As a result membrane conductivity increases. It causes, however, at the same time gradual increase in the distance between the individual polymer chains and thus deterioration of the membrane stability. It makes the membrane also more sensitive to the chemical attack. Therefore, it is necessary to have relevant information on the membrane properties allowing to predict its behaviour and possible critical values of the membrane functionalisation causing break down of its stability. Beside ion exchange capacity important complementary information can also be provided by the ion exchange kinetics. Both these information may be obtained during a single experiment if it is designed properly.
The theory of the ion exchange kinetics is based on the equations of the coupled diffusion in the semi‑infinitive plain represented by the ion selective membrane, Eq. (1).
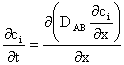 (1)
(1)
Here DAB is the diffusivity of the coupled diffusion in the membrane volume. It is defined by Eq. (2).
![]()
![]() (2)
(2)
In Eqs. (1) and (2) t indicates time, c concentration, z charge number, D diffusivity and subscript i ion A or B.
An important complication, when compared to the rigid inorganic materials, represents the fact that values of diffusivities change with the composition of the pore fluid inside the membrane, i.e. during the ion exchange process itself. In the present study we consider linear dependence of these parameters values on the pore fluid composition given by Eqs. (3) and (4).
![]() (3)
(3)
![]() (4)
(4)
Where yi represents a dimensionless concentration expressed by the Eq. (5).
![]() (5)
(5)
In this equation cx indicates concentration of the ion exchange sites in the membrane. It is assumed, that ion exchange process influences diffusivity of the individual ions on the similar way, i.e. following equations are supposed to be valid.
![]() (6)
(6)
![]() (7)
(7)
Superscript (1) indicates that the membrane is in the cycle of the respective ion indicated in the subscript. Superscript (0) corresponds to the opposite situation, i.e. membrane is in the cycle of the second ion. Variable g in Eqs. (3) and (4) is defined as follows.
![]() (8)
(8)
Under these assumptions following relationship for the fractional attainment of the ion exchange process equilibrium F can be obtained.
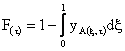 (9)
(9)
Here t is the dimensionless time defined by Eq. (10) and x dimensionless coordinate defined by Eq. (11). Value of yA(x,t) can be obtained by integrating Eq. (12).
![]() (10)
(10)
![]() (11)
(11)
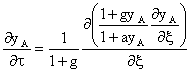 (12)
(12)
Where L is equal to the half of the membrane thickness and a = g-1.
Under an assumption, that one value of diffusivity is known from an independent source, remaining three diffusivity values can be obtained by the analysis of the experimental data. So, the diffusivity value from an independent source remains the only open issue. Suitable option represents diffusivity evaluated from the conductivity value using the theory of the absolute ionic mobility inside the membrane. It is described by the following equation.
![]() (13)
(13)
In Eq. (13) k indicates the Boltzman constant, T absolute temperature, r membrane resistivity (inverse value of the conductivity), F Faraday constant and R universal gas constant. The variable c indicates concentration of the charge carriers in the pore fluid. It can be evaluated according to the following relationship.
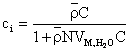 (14)
(14)
Here ![]() represents dry membrane density, C membrane ion exchange capacity, N
number of water molecules absorbed by the membrane per an ion exchange
site and
represents dry membrane density, C membrane ion exchange capacity, N
number of water molecules absorbed by the membrane per an ion exchange
site and ![]() than partial molar volume of water.
than partial molar volume of water.
The problem of determining the membrane conductivity at the required conditions was already discussed. Let's turn the attention to the experimental evaluation of the ion exchange kinetics. In order to evaluate diffusivity of the ions participating in the ion exchange process the data from the course of the ion exchange have to be recorded. The concentration of the released ions is subsequently transformed to the F defined by Eq. (15). It is then plotted in dependence on the square root of the dimensionless time t. The linear part of the dependence corresponding to the diffusion in the semi-infinite plain is fitted by the straight line. Slope of this line is needed for the both directions of the ion exchange, i.e. cases of ion A or B being primary inside the membrane have to be evaluated.
![]() (15)
(15)
The variable n indicates the molar amount of the exchanged ions released by the membrane into the solution.
In the classical case of the exchange of proton for the alkali metal ion the typical method of determining the amount of protons released by the membrane sample represents an acidobasic titration. It is, however, not a suitable option in the present case. This is because of its relatively low sensitivity at the early stages of the ion exchange process. Moreover, for some ions this method of quantitative analysis is rather difficult.
In an optimal case on‑line analysis is used. This is rather simple for the membrane in the proton cycle. In such a case proton released by the membrane is easily monitored by the glass pH electrode. An attention has to be paid to the stability and sensitivity of the electrode. An example of the proton concentration course in the solution during the ion exchange process recorded by the means of the glass electrode is shown in Fig.2A. Course of the evaluated dimensionless transition function is than shown in Fig. 2B.
In the case of the membrane being at the beginning of the experiment in the sodium cycle the situation is more complicated. On‑line analysis is hardly possible and samples have to be collected in the defined time intervals. The sodium ion content has to be analysed subsequently by the means of a suitable experimental technique. ICP, polarography or ion selective electrode represent typical examples of the analytical techniques applicable. The transport parameters of the ions inside the membrane have to be fitted according to the experimentally determined slopes of the transition functions. On this way the membrane transport properties are obtained. Parameters g and dprovide an additional qualitative information on the membrane properties. It concerns especially the role of the electroosmosis in the mass transfer. At the same time the information on the free membrane volume can be obtained. This allows deeper insight into the membrane structure in the swollen state and into the role of the individual mass transport mechanisms inside the membrane.
|
|
|
|
FIG. 2: On‑line analysis of the ion exchange process. Exchange of proton being originally present in the membrane for sodium ion; material: sulphonated polyphenyleneoxide, degree of sulphonation 43 %, 0.1 M NaCl solution; pH measured by the Ross pH electrode (Orion). A ‑ course of the concentration of protons in the solution in time; B ‑ dimensionless transition function evaluated from the concentration course. |
|
Selected membranes showing the most promising properties are subject of testing in the laboratory fuel cell in order to provide comparison of theirs performance with the standard material, typically prefluorinated sulphonated membrane, under the fuel cell conditions. In order to avoid unexpected side effects, several conditions have to be satisfied. The first of them represents application of the suitable gas diffusion electrode. If the fuel cell is working at the temperature below 100 °C, application of the commercial electrode ensures negligible distortion of the data by the irreproducible electrode performance. However, the situation is different in the case of the novel ionomers being tested with respect to theirs performance at the temperature above 100 °C. Perfluorinated sulphonated polymer used typically to impregnate the catalyst layer is loosing its performance at such a high temperature. This is because it looses the water. Therefore method of electrode impregnation has to be modified. In an optimal case it is impregnated by the ionomer identical with this constituting the membrane. Humidification of the gasses fed has to be carefully controlled to avoid any artefacts caused by the disturbance of the fuel cell water management. In order to provide the most reproducible cell assembly, single cell is typically used at this stage. Dimensions of the cell are subject of optimisation. To small cell doesn't allow precise process control. At the same time to large dimensions cause disturbances in the homogeneity of the gasses distribution along the electrodes surface. At the same time, using a single cell, the electronic load typically can't control process at the high current load due to the low cell voltages. Two types of characteristics are typically determined during the fuel cell testing. The first, more rapid one, is a load curve shown in Fig. 3A, i.e. current corresponding to the specific cell voltage. These characteristics can be divided into three different regions. In the first one the cell performance is controlled by the electrode reaction activation overpotential. The second one corresponds to the performance being controlled by the ohmic resistances in the system. The last one belongs to the domain of the mass transfer control. For the characterisation of the membrane suitability for this type of application is decisive the second region, because the membrane represents an electrolyte, i.e. ohmic element. Using cell voltage and corresponding current load, cell performance can be evaluated. It is shown in Fig. 3B. Using the results of this analysis, optimal conditions of the long term testing can be determined. Theoretically they correspond to the cell maximum power output. In practice, however, slightly lower current load is used. This is due to the possible changes of the fuel cell performance in time, more sensitive process control and reduced danger of the cell flooding.
|
|
|
|
FIG. 3: (A) Load curve of the fuel cell, (B) cell performance in the dependence on the current load; H2/O2 fuel cell, temperature 50 °C, Nafion 117 membrane, E-TEK commercial gas diffusion electrodes, 5 g Pt m-2. |
|
The aim of this contribution is just to provide a brief survey of the selected electrochemical methods used for the characterisation of the ion selective membranes with a focus on theirs perspective application in the fuel cell technology as a solid polymer electrolyte. This is just a complementary text, which is, due to the space limitation, not able to provide detail information. This is the subject of the talk delivered during the summer school.
.
JOÃO G. CRESPO
Requimte / CQFB - Department of Chemistry, Faculty of Science and Technology, Universidade Nova de Lisboa, Campus da Caparica, 2829-516 Caparica, Portugal
email: jgc/dq.fct.unl.pt
In many situations the focus of innovation does not lie on the search for novel structures for active compounds, but on the optimization of production processes. The challenge today is also to concentrate engineering research in non-traditional reaction processes, using new synthetic methodologies such as microreactor technology, solventless reaction, solid-phase chemistry, and integrated reaction and product recovery. The same holds true for the design of new separation approaches: reactive distillation and crystallization, fluid extraction using new (neoteric) solvents with a greener character, and membrane processing, among others.
It is clear nowadays that many key areas concern the use of novel materials and solvents, and the integrated use of both. Advances in nanotechnology will enable the solution-oriented design of nanomaterials - stationary phases for chromatography, membranes with tuned selectivity, self-cleaning capabilities and extended life-time, nanoparticles, nanoassemblies and other custom-designed nanostructures.
Water is the most widespread solvent in nature and, without question, the most important for humankind. However, most reactions and chemical processes employ organic solvents that are usually volatile, flammable, toxic and hazardous, i.e. incompatible with the principles and aims of green chemistry [Anastas and Warner, 1998]. The search for replacement of classical organic solvents is therefore one of the most active fields of contemporary chemistry. Various fluids, including supercritical fluids, perfluorinated liquids and room temperature ionic liquids are being investigated as green alternatives to classical organic solvents.
This lecture discusses the integration of neoteric solvents - such as supercritical fluids, fluorinated solvents and room temperature ionic liquids - with membranes, aiming the design of new processes combining the potential of these solvents with the unique properties of membrane materials.
In what concerns the use of supercritical fluids (SCF) this lecture will discuss new ideas recently proposed in the field of SCF [Osuna, Serbanovic and Nunes da Ponte, 2005] and membrane coupled processes [Sarrade, Guizard and Rios, 2002]. The first issue to be discussed concerns the recovery of CO2 by membranes during a supercritical fluid extraction process. This procedure aims enhancing the extraction process by lowering the energy required for recycling CO2. The use of supercritical CO2 extraction coupled with membrane nanofiltration is also discussed, as well as its use for purification of low molecular weight compounds. This new process has been proposed as a solution allowing for the recovery of the product(s) of interest at high flow rates, with a good control of the whole extraction/separation process and reduced energy consumption. Cross-flow filtration applied to viscous liquids (namely, room temperature ionic liquids) fluidified with supercritical carbon dioxide constitutes another interesting example. The basic idea of SCF assisted membrane filtration is to reduce the liquid apparent viscosity at ordinary temperature by injecting a gas (CO2) under supercritical conditions before filtration. Finally, it will be discussed the preparation of solvent-free membranes - extremely interesting for medical applications - using a CO2 assisted phase inversion method. The effect of the solvent affinity and depressurization rate on the morphology and resulting performance of the membranes will be also discussed [Temtem, Casimiro and Aguiar-Ricardo, 2006].
The use of perfluorinated hydrocarbons, represented by perfluorohexanes (FC-72TM), in synthesis as solvent had been quite limited until recently. Perfluorocarbons are generally immiscible with water and organic solvents at room temperature except for some low molecular weight solvents. However, there are several cases in which a mixed solvent system composed of perfluorinated solvents and other organic solvents becomes homogeneous on heating. This thermomorphic behaviour in a fluorous/organic mixed system was applied by Horváth and Rábai, 1994, which achieved facile separation of catalysts and products in Rh-catalyzed hydroformylation of alkenes [Horváth and Rábai, 1994]. The use of perfluorocarbon solvents in liquid membrane systems has been proposed and described, but full exploitation of this approach is still in its infancy.
Room Temperature Ionic Liquids (RTILs) are a special class of molten salts that are fluid at room temperature and possess relatively low viscosity (when compared with some of the traditional molten salts). Although the list of new RTILs grows very rapidly, most of them are based on cations of tetraalkyl ammonium and phosphonium salts and hetero-aromatics, typically associated with inorganic and organic anions such as BF4, PF6, N(CF3SO2)2, CF3SO3, RCO2, NO3, ClO4, etc. These liquids possess physical-chemical properties that qualify them as "tailor-made" green solvents for various reactions and processes [Dupont, 2005].
Based on these properties a number of new processes have been proposed integrating RTILs and membranes. This lecture will discuss some of these new approaches: 1 - development of RTILs supported liquid membranes and membrane contactors for liquid extraction [Schäfer et al., 2005] and gas/vapour permeation; 2 - multiphase catalysis employing RTILs, including biocatalytic processes, making use of the capabilities of the membrane as a permeselective barrier and/or as a catalytic compartment; 3 - development of new membrane materials, doped with RTILs, for use as new electrochemical devices and sensors. This lecture will also address the use of different techniques for characterization of the membrane interaction with RTILs, namely employing impedance spectroscopy, X-ray photoelectron spectroscopy and Raman confocal spectroscopy.
References
P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, New York, 1998.
A. B. Osuna, A. Serbanovic and M. Nunes da Ponte, Supercritical Fluids, in: Green Separation Processes, Edited by C. A. M. Afonso and J. G. Crespo, Wiley-VCH, Weinheim, 2005.
S. Sarrade, C. Guizard and G. M. Rios, Desalination, 2002, 144, 137.
M. Temtem, T. Casimiro and A. Aguiar-Ricardo, Journal of Membrane Science, published electronically in 2006.
I. T. Horváth and J. Rábai, Science, 1994, 266, 72.
J. Dupont, Ionic Liquids: Structure, Properties and Major Applications in Extraction / Reaction Technology, in: Green Separation Processes, Edited by C. A. M. Afonso and J. G. Crespo, Wiley-VCH, Weinheim, 2005.
T. Schäfer, L. M. Branco, R. Fortunato, P. Izák, C. M. Rodrigues, C. A. M. Afonso and J. G. Crespo, Opportunities for Membrane Separation Processes Using Ionic Liquids, in: ACS Series on Ionic Liquids III: Fundamentals, Progress, Challenges, and Opportunities, Edited by. R. D. Rogers and K. R. Seddon, 2005.
A. FIGOLI
Research Institute on Membrane Technology, (ITM-CNR), c/o University of Calabria, Via P. Bucci 17/C
87030 Rende (CS), Italy (a.figoli/itm.cnr.it)
New concepts to produce innovative polymeric membranes for a broad range of industrial applications, such as cosmetic, pharmaceutical and chemical, are described in this work. In particular, two novel membranes, such as a) the multilayer membrane and b) the polymeric capsules prepared by phase inversion technique, were investigated.
The idea of this new multilayer film is presented in figure 1.
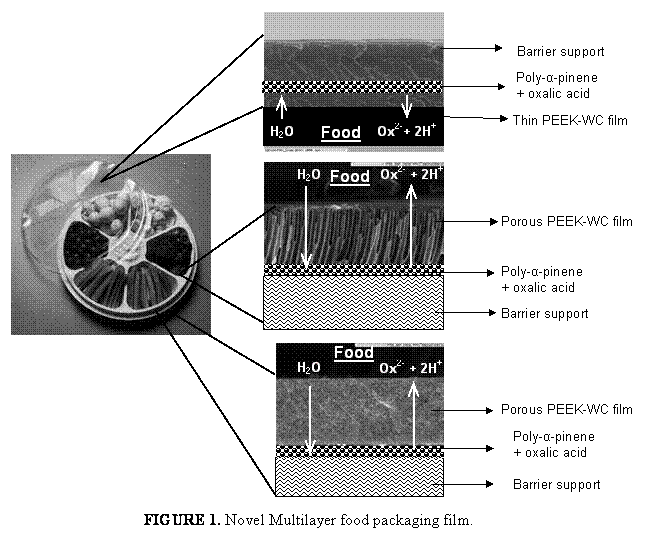
The multilayer film is made of three layers:
a) An outer dense layer to control the exchange rate of gases and vapour between the external and internal environment of the food packaging
b) An intermediate adhesive tie-layer which has also the function of reservoir of antimicrobials.
c) A porous third layer, made by phase inversion, which is able to control the release of antimicrobials to the food in time. The release of antimicrobials can be adjusted changing the morphology of the porous layer. The morphology can be controlled varying the phase inversion process conditions.
The multilayer films were prepared as shown in figure 2. All separate layers of the multilayer film could be cast subsequently on one another without removing the dense film from the glass substrate.
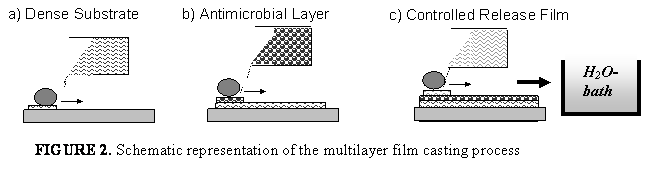
a) The dense (80/20) PEEK-WC/p-α-p layer, used as substrate for the multilayer film, was prepared by casting a solution of 10 wt% 80/20 PEEK-WC/p-α-p in chloroform with a hand casting knife fixed at 250 μm. A dense 80/20 PEEK-WC/p-α-p film remained on the clean glass substrate after the solvent was evaporated [3].
b) The second layer made of poly-α-pinene with and without oxalic acid (0,5, 10 and 25 wt%), was stirred at 70°C and cast at 0% RH and 70°C in a climate chamber (Angelo Antoni, Aquile 302 Challenge). The handcasting knife was fixed at 100 μm. Directly after casting the formed double layer film was removed from the climate chamber and allowed to cool at room temperature.
c) A porous PEEK-WC film was then cast on the double layer film previously prepared. The porous layer was prepared by dry-wet phase inversion. A casting solution was prepared with different concentrations of PEEK-WC in N,N diethylacetamide (DMA) (15, 19 and 23 wt%). The films were cast in a climate chamber at 50% RH and 20°C with the hand casting knife fixed at 350 μm. The porosity and morphology of each membrane were varied changing the time of exposure to air before precipitation (45s./240s.) and the water-bath temperature (0°C and 40°C) [4-7]. The multilayer film was removed 5 minutes after immersion from the coagulation bath and dried overnight at room conditions.
The preparation and characterization of the single layers which makes the multilayer, and the whole multilayer film, was deeply studied. The single layers and the multilayer film were characterized for better understanding the gas/water transport and mechanical properties through the film itself. The gas properties of the multilayer film are similar to the single dense layer. This means that the other two layers do not affect the gas transport through the multilayer film. It was also observed during the mechanical test that the multilayer film broke in two separate steps. The porous film breaks first while the dense remains operational after the break.
The cross sections of the final antimicrobial multilayer films was examined by SEM (figure 3). The individual layers of the film, indicated by the arrows, can clearly be distinguished from the reported images.
Furthermore
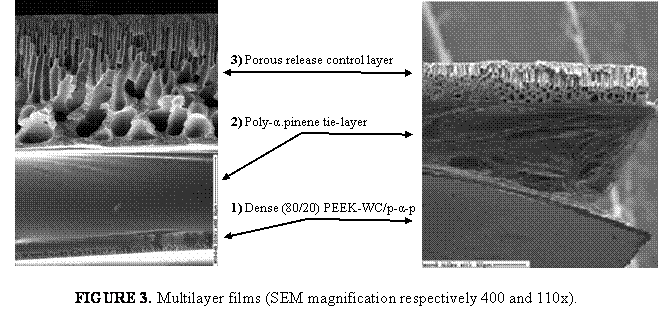 |
It was proven that the release rate of antimicrobials from the multilayer films depended strongly on the phase inversion processing conditions of the third porous layer. The release of oxalic acid increases mainly with decreasing the coagulation bath temperatures. The release rate of antimicrobials can be modulate changing the morphology of the porous layer.
b) Micro-capsules using a membrane process combined with phase inversion technique was exploited [8]. This technique can be identified as an integration between the traditional chemical capsule techniques (coacervation or phase inversion) [4,5,7] and the mechanical capsule technique (pressure extrusion).
This technique permits the formation of monodispersed polymer (modified polyetheretherketone) macrocapsules with different morphologies. The capsule morphology, porosity, size and shell thickness is easily adjusted changing the ingredient parameters such as polymer concentration, solvent and non solvent involved phases in the process. In particular, the prepared capsules show a size diameter from 200 to 1200 micron.
Active compounds, such as antimicrobials, active carbon, are loaded in the capsules during its production, which are then employed for preparing a well-defined capsule containing film. Such film has been prepared dispersing the capsules in a polymeric solution, which has just the function of bounding the capsules, and then cast it, for example, on a traditional food packaging film such as PET.
The capsules, in contact with the food environment, release the antimicrobial agent in a controlled way thanks to the different morphology and shell porosity. Experiments of antimicrobials release are reported as function of the different membrane morphology. Antimicrobials agents such as oxalic or ascorbic acid have been employed.
The experimental capsule dimensions have been compared with the droplet diameter obtained by balance force analysis along the pore month. The force balance analysis, along the droplet contact line, has allowed to understand the droplet detachment mechanism in function of pore diameter, membrane hydrophobicity, polymeric solution and process parameters.
In Figure 4, the SEM pictures of the made PEEKWC capsules are presented. These capsules are obtained with pore membrane diameter of about 500 mm, using the same polymer solvent (DMF) and non-solvent (water).
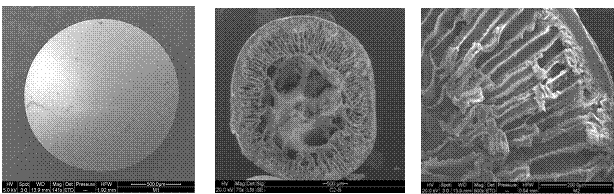
Figure 4. SEM pictures of different prepared capsules using a film with the pore size of about 500 mm using as phase 1, PEEKWC/DMF 8 wt.%; phase 2, dodecane and phase 3, water.
The formation of mono-dispersed PEEKWC capsules with different morphologies has been carried out. The capsule morphology and dimension can be easily adjusted changing the involved phases (phase 1, 2 and 3) in the process and the membrane pore sizes respectively. These preliminary tests, using membrane pore diameter ranging from 500 mm to 1000 mm, show that droplet sizes are almost constant. Furthermore, computational studies on the controlled release of active chemical compounds in function of capsule morphology and dimensions are in progress. This study can be considered as the starting point toward the production of loaded nano-sized capsules.
References
[1] J.Jansen, Master Thesis work: "Preparation and Characterisation of a New Antimicrobial Polymer film for food Packaging Application" performed at Institute on Membrane Technology (ITM-CNR), Italy (A. Figoli, E. Drioli) in collaboration with the Membrane Technology Group, University of Twente, The Netherlands, (M.Wessling).
[2] A. Figoli, E. Drioli, J.Jansen, M.Wessling, Film antimicrobico per prolungare la shelf-life, Rassegna dell'Imballaggio, ISSN0033-9687, Nov. 2004, n.16, Year 25°.
[3] A. M. Torchia, G. Clarizia, A. Figoli, E Drioli, Potentiality of PEEWC as new material in food packaging, Italian Journal of Food Science, 16 (2004) 173-184.
[4] J.C.Jansen, M.G.Buonomenna, A.Figoli, E.Drioli, Asymmetric membranes of modified poly(ether ether ketone) with an ultra-thin skin for gas and vapour separations, Journal of Membrane Science, 272 (2006) 188-197.
[5] M.G. Buonomenna, A. Figoli, J.C. Jansen, M. Davoli, E. Drioli, Mat. Res. Soc. Symp. Proc., Membranes, Preparation, Properties and Applications, 752 (2003) 3-8.
[6] Han M-J, Effect of Propionic Acid in the Casting Solution on the Characteristics of Phase Inversion Polysulfone Membranes, Desalination, 121 (1999) 31-39.
[7] Buonomenna M.G. Figoli A. Jansen J.C. Drioli E., Preparation of Asymmetric PEEKWC Flat Membranes with Different Microstructures by Wet Phase Inversion, Journal of Applied Polymer Science, 92 (2003) 576-591.
[8] W. Bakker et al., Patent WO2004/096405, Akzo Nobel B.V., 2004.
M. WESSLING
University of Twente, Membrane Technology Group, 7500 AE Enschede, The Netherlands
Progress in micro and nanotechnology enables the material scientist to design membrane architectures having very specific functions. Off course, not the membrane is smart, but the designer has used his smartness and creativity to prepare these new membranes. Often such new membranes and membrane functions evolve at the interface of different disciplines. The membrane architect gets impulses for new ideas when discussing with scientist from unknown research communities.This presentation will give four examples where membranes are not used in its classical applications, but at interfaces with other disciplines like microfluidics, emulsification, tissue engineering and biomimetic surfaces..
In this article we present a new versatile replication method to produce thin polymeric microfluidic devices with tunable porosity. This method is based on phase separation of a polymer solution on a microstructured mold. Compared to existing microfabrication techniques, such as etching and hot embossing, our technique offers four advantages: (a) simple and cheap process that can be performed at room temperature outside clean room facilities; (b) very broad range of applicable materials (including materials that could not be processed before); (c) ability to make thin flexible chips; (d) ability to introduce and tune porosity in the chip. By introducing porosity, the channel walls can be used for selective transport of gasses, liquids and solutes. A proof-of concept will be given, by showing fast CO2 transport through the channel walls of a porous polymer chip. Furthermore, it will be demonstrated that the gas permeation performance of chips can be enhanced dramatically by a decrease in chip thickness and incorporation of porosity. We expect that the development of porous chips can lead to the on-chip integration of multiple unit operations, such as reaction, separation, gas liquid contacting and membrane emulsification.
The same microfluidic chips described above can be used to prepare mono-dispers droplets. The continuous phase permeates from the porous chip into the channel. Droplet breakup in the channel occurs in a very controlled manner and the size of the channel determines the size of the droplet. To produce oil-in-water or water-in-oil droplets one must change the surface properties of the porous chip.
Scaffold design is a major theme in tissue engineering; an optimal design of a scaffold appears to be crucial. Two topics should be addressed profoundly; i.e., porosity and micro-patterning of the surface. Adequate porosity ensures sufficient nutrient diffusion to the cells and micro-patterning induces alignment of the cells and therefore organizates in vitro tissue to mimic in vivo tissue. Currently, fabrication of scaffolds does not associate porosity with micro-patterning. This paper reports a novel one-step method to fabricate porous micro-patterned 2-D scaffolds with tunable porosity and pore-morphology to acquire adequate nutrient diffusion and with incorporated a micro-pattern to align the cells. Scanning electron microscopy showed that the poly(l-lactid acid) scaffolds have high porosity and pore-interconnectivity and subsequently, that the various micro-pattern designs imprinted in the scaffold are of excellent quality. Diffusion of glucose through the scaffold was high, indicating the scaffolds are successful with respect to nutrient diffusion. HUVEC culturing proved the scaffolds are suitable for cell culturing. More extensive experiments with culturing mouse myoblasts, C2C12, showed tissue organization could be controlled; the micro-pattern design affects the extent of cell alignment. The 2-D scaffold design appears to be very effective and is very promising as basis for 3-D structures due to the combination of nutrient diffusion through the porous scaffold and induced cell alignment controlled by the micro-pattern.
Phase Separation Micro Molding is utilized to prepare surfaces of a hydrophobic material with a hierarchal roughness, mimicking the self-cleaning properties of the lotus plant leaves. First of all, the microstructure increases the roughness of the surface, and additionally the porosity of the microstructure superimposes a roughness on a smaller length scale. The hydrophobicity of Hyflon ® AD was optimized by variation of the patterns of the microstructure and the phase separation process, tuning the roughness on both levels. The optimized surfaces, in terms of hydrophobicity, display water contact angles well above 160 degrees. Due to the very low contact angle hysteresis, water droplet roll-off angles are as low as 0.5 degrees.
References
(1) J. de Jong, B. Ankone´, R. G. H. Lammertink* and M. Wessling, New
replication technique for the fabrication of thin polymeric
microfluidic devices with tunable porosity, Lab Chip, 2005, 5,
1240-1247
(2) Bernke J Papenburg; Laura Vogelaar, Dr; Lydia A Bolhuis-Versteeg; Dimitrios Stamatialis, Rob G Lammertink, Matthias Wessling, One-step Fabrication of Porous Micro-patterned Scaffolds to Control Cell Behavior, submitted to Biomaterials
(3) Laura Vogelaar, Rob G. H. Lammertink,* and Matthias Wessling, Superhydrophobic Surfaces Having Two-Fold Adjustable Roughness Prepared in a Single Step
Langmuir, 22 (7), 3125 -3130, 2006