PC 02
DESIGN AND SYNTHESIS OF NANOSTRUCTURED MULTIBLOCK COPOLYMERS WITH OPTICAL PROPERTIES
A.-L. Roya,c, B. Chuonga, M. Savea, B. Charleuxa, D. Krehera, A.-J. Attiasa*, F. Kajzar b*, I. Rau b
a : Laboratoire de Chimie Macromoléculaire, Université Pierre et Marie Curie
UMR CNRS 7610, 4 Place Jussieu, case 185, F-75252, Paris Cedex 05, France
b :Laboratoire Cellules et Composants, Commissariat à l'Energie Atomique,
Centre de Saclay, 91191 Gif-sur-Yvette Cedex, France
We are particularly interested in the synthesis of new push-pull chromophores, constituted of a thiophene or bipyridine -conjugated bridge, and organometallic or organic electron-donor and electron-acceptor groups (Fig 1). These chromophores, showing relatively high values up to 7000.10-48 esu, are functionalized or not in order to incorporate them, covalently or not respectively, in polymers.

Fig 1 : Push-pull chromophores
Moreover, the last fifteen years, controlled free-radical polymerization (CRP)[1] methods have proved to be extremely efficient for the synthesis of a variety of new and well-defined macromolecular architectures. As an example, the synthesis of multiblock copolymers by successive polymerization of monomers requires many synthetic steps which increase the probability of side reactions. Consequently, we took advantage of a new strategy recently developed by Otsuka[2] and Koning[3] which consists in the synthesis of a multifunctional macromolecular control agent, in order to allow the controlled insertion of a monomer along the macromolecular chain, producing multiblock copolymers in limited steps number (Fig. 2).
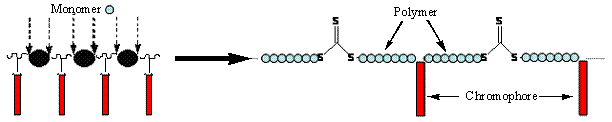
Fig 2 : Insertion of monomer in a multifunctional "NLO-chromophore bearing" macro-RAFT agent.
Consequently, we will shortly sum up our convergent general approach for the synthesis of 6,6'-(disubstituted)-3,3'-bipyridine based chromophores.4,5,6 Then, the polymeric structures incorporating the Disperse Red 19 (as a reference material) with a controlled architecture will be described, promising results being obtained with polystyrene playing the spacer role. Indeed, a well-defined distribution of the chromophores in the polymer is observed by controlled radicalar polymerization using RAFT process, and allows to prevent aggregation between chromophores. Eventually, the replacement of Disperse Red 19 by one of our more efficient NLO chromophore is currently undergoing and if we experimentally evidence the same behaviour, we should present even better NLO activities at a macroscopic level.