1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69
R. LAGA*, A. BRAUNOVÁ, Č. Koňák, M. PECHAR, V. ŠUBR, K. ULBRICH
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovský Sq. 2, CZ-162 06 Prague 6, Czech Republic
*corresponding author; email: richard.laga/gmail.com
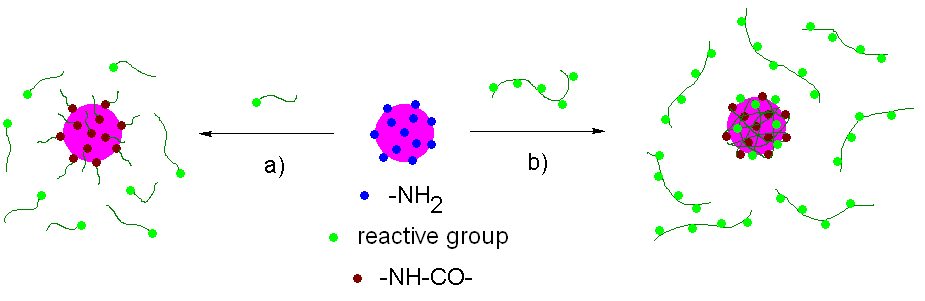
Surface modification of polyelectrolyte complexes (PEC) of DNA and synthetic polycations designed as nonviral vectors for in vivo gene delivery was studied. The PEC were prepared by self-assembly of poly(L-lysine) and DNA at the molar charge ratio +/- = 2:1 in aqueous solutions; the resulting particles carried free positively charged amino groups on the surface. The surface modification (coating) was carried out using new reactive, reductively or hydrolytically degradable polymers based on N-(2-hydroxypropyl)methacrylamide (HPMA) or poly(ethylene glycol) (PEG). Amine-modified polystyrene latex beads (RH ~ 25 nm) were also used for a model study of nanoparticles coating. Particle size (RH, RG) and molecular weight (Mw) were monitored by static and dynamic laser light scattering methods.

Mw and RH values of the complexes as well as those of the latex beads increased immediately after the modification. Subsequently, the size characteristics were stabilized. The modified particles were more stable to aggregation, adsorption of albumin and dissociation in 0.15 M NaCl than the unprotected ones.Incubation of particles coated with reductively cleavable polymer with L-glutathione (GSH) led to the cleavage of disulfide bonds resulting in a slow decrease in Mw and RH followed by aggregation of the particles. Substitution of GSH with uncharged 1,4-dithioerythritol caused the more pronounced decrease in Mw and RH; no aggregation was observed. The hydrolysis of PEC coated with hydrolytically cleavable polymer led to a slight increase in the particle size.
This project was supported by EU grant, GIANT, No. 512087.
J. HAŠEK, J. DOHNÁLEK, J. DUŠKOVÁ, J. KOLENKO, T. SKÁLOVÁ
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
Well defined supra-molecular organization of macromolecules is of principal importance for function of biological systems. Quick and unique folding and clustering of macromolecules into functional units is guarantied by special macromolecules - chaperons. However, in most cases, much simpler molecules can ensure the desired quick and unique folding. A special case of generation of an error-free supra-molecular ordering is protein crystallization. In spite of the fact that there is a number of crystallization protocols with a high performance, the processes of crystallization have not been understood fully yet.
The most frequently used polymers in protein crystallography are poly(oxyethylene) (POE, MW=200-20000), POE monomethyl ether (POE MME) and poly(vinylpyrrolidone). They are very successful precipitants (/1/, pages 7-29) and cryoprotectants (/1/, page 44). Recently, we showed that the polymer additives play an additional important role /2/. They form relatively stable coatings of specific areas on protein surface and thus they embarrass an access of neighboring molecules to large areas in neighborhoods of the polymer anchor leading so (in an optimal case) to the desired unique clustering of macromolecules.
Choice of different supra-molecular orderings can be controlled and the quality of supra-molecular order can be significantly improved by proper choice of low and/or middle molecular weight polymers. The applicability of the polymer additives can be further enhanced by using well defined co-polymers. Review of various basic adhesion modes of POE-type polymers and POE-PE copolymers at the protein surface was described by us in /2/.
Stability of the supra-molecular systems described above is low because they are based on non-covalent interactions only. However, chemical cross-linking using several types of bi-functional spacers can enhance their stability.
/1/ Crystallization Research Tools, Hampton Res., Ca. (2003) 13, 7-44.
/2/ Hašek J., Zeitschrift fur Kristallografie (2006) 23, 613-619.
L. SAMOKHINA, M. BALLAUFF
Department of Physical Chemistry I, University of Bayreuth, Germany; samokhina/uni-bayreuth.de
The interactions of the quenched spherical polyelectrolyte brushes (SPB) with surfactant counter-ions resulting in macromolecule complexes were considered in the present work. It was demonstrated that the surface form affects a complex formation ability of polyelectrolyte chains in the solutions of oppositely charged surfactant ions, i.e. an interaction nature of the surfactant monomers with polyelectrolyte chains grafted to the spherical surface has its own specific as compared with them grafted to the planar surface. Mainly, it appears in a different allocation of the surfactant ions in a brush crown of SPB and guarantees the new unique properties of SPB-surfactant complexes profitable in biotechnological processes. The possibility was assayed to control the properties of complex macromolecules structures via a variation of the functional groups in the polyelectrolyte chains, a surfactant counter-ion type, a charge ratio in different concentration regimes of the spherical nanoparticles.
The combination of scattering and microscopy methods allows to reveal a concentration range of a co-existence of two kinds of aggregates in the solution, namely, the complex nanoparticles with "minimicellar clasters" inside the brush and the ordinary micelles of the surfactant. The influence of the presence of inorganic salts on the properties and stability of prepared compelex nanoparticles was discussed in detail.
S.K. Filippov*a, A.V. Lezov a, S.B. Lesnichin b, A.S. Oliferenko b, E.A. Komarova b, O.Yu. Sergeeva b, N.S. Domnina b
a V.A. Fock Research Institute of Physics, St. Petersburg State University, Uljanovskaja ul.1, 198504, Saint- Petersburg, Russia (S.Filippov/pobox.spbu.ru)
b Chemical Faculty, St. Petersburg State University, University av. 26, 198504, Saint-Petersburg, Russia
Dextrans hydrophobically modified by sterically-hindered phenols represent a novel class of amphiphilic molecules possessing an enhanced biological activity. The hydrodynamic properties of such conjugates in aqueous solution were characterized by a combination of Cryogenic Transmission Electron Microscopy (Cryo-TEM), fluorescent spectroscopy, Atomic Force Microscopy (AFM) and Dynamic Light Scattering (DLS). All solutions investigated contained aggregates (Figure1) the structure, shape, and critical aggregation concentration of which are influenced by amount of the inserted phenol groups in the polymer matrix. It was established that the critical aggregation concentration could be well approximated by a logarithmic function of the substitution degree of the glycoside units.
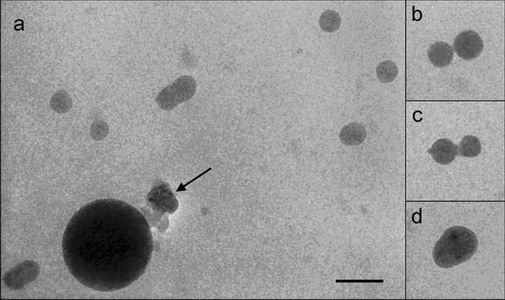
Figure 1. Cryogenic transmission electron microscopy micrographs of D-2 sample (DS=1.9%). Arrow indicates the ice artifacts. Bar indicates 100 nm.
L.Q. AMARAL
Institute of Physics, University of São Paulo, C.P. 66318, São Paulo, SP, CEP 05315-970, Brazil, amaral/if.usp.br
The assembling of hydrated synthetic or natural amphiphiles is studied usually either at small concentrations, up to 1 mM, with emphasis on physico-chemical properties of micelles and vesicles, or at higher concentrations (measured as wt%), where liquid crystalline phases with long range order occur. In this work the structures formed by some charged double-chain amphiphiles are investigated by X-ray in the concentration range 1 mM (where vesicles exist in isotropic solution) up to a few wt%, where in many circumstances vesicles are transforming into other structures:
This work focus on similarities and differences of the structures formed by the different systems under investigation.
(1) K.A.Riske, L.Q.Amaral and M.T.Lamy-Freund, Biochimica & Biophysica Acta 1511 (2001) 297-308.
(2) K.A.Riske, L.Q.Amaral, H.G.Dobereiner and M.T.Lamy-Freund, Biophysical Journal 86 (2004) 3722-3733.
(3) L.Paccamiccio, F.Spinozzi, P.Mariani and L.Q.Amaral (to be published)
(4) E. Feitosa and L.Q.Amaral (to be published)
(Financial support of FAPESP and Laboratório Nacional de Luz Sincrotron)
A. BANC1*, L. NAVAILLES1, D. RENARD2, Y. POPINEAU2, C. MANGAVEL2, B. DESBAT3, T. BUFFETEAU3
1 CRPP, CNRS UPR 8641, Avenue A. Schweitzer, 33600 Pessac, France (banc/crpp-bordeaux.cnrs.fr)
2 BIA, INRA Centre de Nantes, BP 71627, 44316 Nantes Cedex 3, France
3 LPCM, Université Bordeaux 1, 33405 Talence cedex, France
Seed storage proteins are important constituents of cereal due to their nutritive and technological properties. These proteins naturally serve as amino acids source for the future development of plants, and are stored in protein bodies, located in the endosperm cells of the seeds. Protein bodies are micrometer-sized organelles emerging from endoplasmic reticulum membranes and may contain up to 80% proteins. The mechanisms leading to the organisation of storage proteins into protein bodies are not well understood. The aim of this project is to identify physical-chemistry parameters which participate in this natural self-assembly. For this study, we worked on a model protein, g-gliadin, a wheat prolamin distinctive by its insolubility in water and its amphiphilic character.
First, we investigate protein-membrane interactions using bio-mimetic systems made of lipidic lamellar phases (C12E5/hexanol/water, PC/water). These kinds of systems enable us to encapsulate proteins between bilayers where confinement may be controlled depending on water content (from 1 to 15 nm). Fluorescence Recovery After Photobleaching (FRAP) experiments, performed on oriented samples, enabled us to measure diffusion coefficients of g-gliadin encapsulated in the lamellar phases at different confinement. The results would suggest strong interactions between g‑gliadin and neutral synthetic model membranes whatever the confinement.
Secondly, in order to precise the nature of the interaction, we carried out experiments on g-gliadin adsorbed at the air-water interface and g-gliadin injected beneath a DMPC monolayer, using three complementary approaches: infrared spectroscopy (PMIRRAS), surface pressure measurements and Brewster angle microscopy. We confirm that g-gliadin is very stable at the air-water interface and we show various secondary structures according to the surface pressure. Injected under a neutral lipid monolayer, g-gliadin adopts various behaviours with respect to the lipid surface pressure. At a natural membrane surface tension the g-gliadin membrane interaction is confirmed with an unexpected "blinking" interaction.
The influence of various physical-chemistry parameters (charge and fluidity of the membrane, [Ca++]) on the nature of the interaction will also be discussed.
A.V. SyBACHIN, A.A. Efimova, E.A. LITMANOVICH, A.A. Yaroslavov
Chemistry Department, Moscow State University, 119992, Leninskie Gory, Moscow, Russian Federation (sybatchin/mail.ru)
Adsorption of a cationic polymer on the surface of mixed liposomes, composed of neutral and anionic lipids, has been examined. The content of anionic lipid (Q) was varied from 10 up to 40 mol.%, these molecules being uniformly distributed within each membrane leaflet and between them. The interaction was accompanied by neutralization of the surface charges of liposomes and their aggregation. The amounts of polycation, required for the complete charge neutralization and formation of the largest particles, was linear in Q, thus indicating that all anionic lipid molecules, from both membrane leaflets, were involved in electrostatic binding with cationic units of the adsorbed polymer.
An excess of the polycation, over needed for the complete neutralization, could be adsorbed on the liposomal membrane. Importantly, the amount of the abundant polymer was independent on the Q value. The PEVP-to-liposome binding was likely terminated when a charge brought in by adsorbed PEVP reached a certain positive value.
Due to lateral segregation and transmembrane migration of lipids, induced by adsorbed polycation, the composition of the interfacial polycation-membrane complexes was the same for all the studied liposomes. A thickness of a layer formed by the adsorbed polycation was found to be approx. 30 mn.
A.A. Efimova, A.A. Yaroslavov
Chemistry Department, Moscow State University, 119992, Leninskie Gory, Moscow, Russian Federation (ephimova/genebee.msu.su)
Interaction of small unilamellar liposomes, composed of anionic cardiolipin (CL) and neutral dipalmitoylphosphatidylcholine (DPPC), with poly(N-ethyl-4-vinylpyridinium) cation (PEVP) has been studied by means of photon correlation spectroscopy, microelectrophoresis, and fluorescence technique.
Binding of PEVP to liquid liposomes whose membranes are in the fluid state leads to significant structural rearrangements in the liposomal membrane: lateral lipid segregation, acceleration of transmembrane lipid migration, etc. However, the integrity of the membrane retains unchanged. The interaction is electrostatic in nature: the polycation is completely removed from the membrane when increasing salt concentration or by addition of a polyanion excess. The removal of polycation restores the initial distribution of lipids in the lateral and transmembrane directions.
In contrast to that, adsorption of PEVP on the surface of solid liposomes (their membranes are in the gel state) produces defects in the liposomal membranes. This makes the complexation irreversible: neither salt nor polyacid are able to remove polycation from the complex.
Importantly, the defects remain when transferring solid liposomes, covered by PEVP, to the liquid. Moreover, the reversible contact of PEVP with liquid liposomes becomes irreversible as PEVP-bound liposomal membranes are converted to the gel state.
The observed phenomena demonstrate a strong dependence of the structure and properties of polycation-liposome complexes on the phase state of the lipid membrane.
D.A. Davidov1, A.A. Efimova1, A.A. Yaroslavov1, F.M. Menger2
1School of Chemistry, Moscow State University, 119992, Leninskie Gory, Moscow, Russian Federation (davidov_dimitri/bk.ru)
2Department of Chemistry, Emory University, Atlanta, USA
Interaction of synthetic polycations with mixed liposomes, composed of neutral phosphatidylcholine and a negative surfactant, was examined. The latter was represented by one-tailed benzyldodecyl phosphate (1), or a gemini surfactant in which two dodecylphosphate residues were bound together through benzyl (2) or cis-stilbene (3) or trans-stilbene (4) spacer. The quasi-elastic light scattering and fluorescence data showed that in all cases addition of polycation solutions to a liposome suspension was accompanied by enlargement of particles in the system and a decrease in the fluorescence intensity of the label incorporated into the liposomal membrane, indicating polycation/liposome complex formation. The stability of the resulting complexes in water salt media was strongly dependent on the chemical structure of surfactants. A NaCl concentration, required for complete dissociation of the complexes, increased as follows: 1 < 2 < 3. The polycation could be also removed from the complexes by addition of excess of a competing polyanion. A salt solution or a polyanion excess also induced a removal of polycation from its complex with 4; however the diameter of particles, deprived of polycation, was as twice as higher then that of the original liposomes. In this case polycation-induced fusion of liposomes likely occurred.
Binding of polyelectrolytes with three-component neutral liposomes, composed of phosphatidylcholine and equal amounts of anionic and cationic lipids, was also explored. Such liposomes were found not to aggregate at any concentrations of polyelectrolytes in the system.
Possible structures of polyelectrolyte-liposomes complexes are discussed.
YU. K. VALEEVA, N.A. ALEKSANDROVA, M.B. GOTTIKH, T.YU. DORODNYH, N.S. MELIK-NUBAROV
Chemistry department, M.V. Lomonosov Moscow State University, Leninskie gory 1-3, Moscow, Russia, 119992 ukava/yandex.ru
The main obstacle for the application of all gene therapeutic approaches consists in the extremely low permeation of these substances across biological membranes.
One of the most promising approaches for the delivery of oligonucleotides (ODN) consists in their complexation with polycations. The application of polycations in practice is limited because of their toxicity and low ability to disturb cell membranes and facilitate ODN permeation into cytoplasm. The present work is focused on the design and synthesis of novel polymeric carriers consisted of polycationic and hydrophobic blocks, which are able to interact with ODNs via electrostatic forces and perturb cell membranes due to insertion of their hydrophobic block.
Block copolymers of Pluronics (three block copolymers of ethylene oxide (EO) and propylene oxide (PO) and N,N-dimethyl-aminoethylmethacrylate (DMAEMA) were synthesized via atom transfer radical polymerization (ATRP). Pluronics L61, P85 and F127 differed by molecular masses and the ratios of EO and PO units were used as macroinitiators to obtain copolymers of DMAEMA with average degree of polymerization of cationic block varied from 15 to 100.
The obtained polymers were able to form stable complexes with ODNs of different structure even in the salt solutions with physiological ionic strength as assessed by fluorescent spectroscopy.
Interaction of the copolymers with erythrocytes caused membrane permeabilization, which indicated their high membrane activity, suggesting that the copolymers of DMAEMA and Pluronics can be applied to facilitate oligonucleotides delivery into a cell.
The work was supported by Russian Foundation for Basic Research (grant №06-03-32403) and European Commision Project PROTHETS LSHC-CT-2004
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovský Sq. 2, CZ-162 06 Prague 6, Czech Republic
*corresponding author; email: mhruby/centrum.cz
Biodegradability of disulfide bonds with glutathione is a very interesting property that can be employed in the design of drug, protein or DNA delivery systems. The extracellular level of glutathione is much lower (ca 4.5 mmol L-1) than the intracellular level (0.1 - 10 mmol L-1 depending on the tissue type) and this fact may be used in designing systems that are relatively stable during transport outside the cell and release the active compound after internalization into the target cell. Poly(adipoyl chloride-alt-cystamine) nanoparticles covalently coated with poly(ethylene oxide) (PEO; Mw = 2000 and 5000) were synthesized by polycondensation and subsequent self-assembling of the polymer in water. They serve as a model system for glutathione-degradable drug, protein or DNA delivery systems. The hydrophobic polyamide forming the micellar core contains disulfide bonds in the main chain and thus its degradation products are of low molecular weight. The degradation of the nanoparticles by reaction with glutathione in the reduced or oxidised forms was monitored by measuring light scattering intensity at 37 °C in media modelling intracellular environment. In this way, the lifetimes (tlf) of the nanoparticles were determined. The reductive degradation of the nanoparticles containing PEO block ofMw = 2000 (Rh = 44 nm) by the reduced form of glutathione was an order of magnitude faster (tlf = 8.4 h) than their degradation with the oxidised glutathione (tlf = 43 h) or spontaneous hydrolytic degradation (tlf = 99 h). The corresponding lifetimes for the nanoparticles containing blocks of PEO of Mw = 5000 (Rh = 60 nm) are similar. We propose a mechanism of the nanoparticle degradation that involves reversible dissolution of the micelle-like nanoparticles into unimers that subsequently undergo irreversible chemical degradation with glutathione.
Financial support of the Grant Agency of the Academy of Sciences of the Czech Republic (grants A100500501, A4050403 and A400480616) and of the Ministry of Education, Youth and Sports (Research Centers Program, grant IM 463 560 8802) is gratefully acknowledged.
P. Chytil, T. Etrych, Č. Koňák, K. Ulbrich
Institute of Macromolecular Chemistry Academy of Sciences of the Czech Republic, Heyrovského Sq. 2, 162 06, Prague, Czech Republic; (chytil/imc.cas.cz)
Water-soluble N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers containing anticancer drug doxorubicin bound via pH-sensitive hydrazone bond exhibit a significant therapeutic effect in the treatment of EL4 lymphoma in mice (1,2). While hydrazone bond is relatively stable in neutral pH (simulation of conditions during transport in blood vessels), the drug is released in mild acid environment (simulation of the environment of endosomes of tumour cells). It was shown that high-molecular-weight conjugates can be more effectively accumulated in solid tumours by the EPR (enhanced permeability and retention) effect than that of lower molecular weight (3). Therefore, micellar carriers are promising candidates for specific delivery of anticancer drugs to solid tumours. In this study we present synthesis and physico-chemical characterization of "hydrazone" conjugates containing hydrophobic substituents (HS) in the polymer structure (4). In addition to the fact that these conjugates formed high-molecular-weight polymer micelles, HS could interact with biological membranes of tumour cells rendering the transport of the conjugates into the cells more efficient.
We used different structures and contents of HS for preparation of HS-bearing polymers according their hydrophobicity (dodecyl, oleoyl, cholesteryl), shape (linear, planar) and also type of linkage between HS and polymer carrier. Hydrodynamic radii of micelles in physiologic solution were about 6 - 18 nm and size distributions were relatively narrow. Also apparent molecular weights of these micelles were in range of 0.5 - 3.5·105, while molecular weight of single polymer chains was about 2·104. The higher was the content and type of HS, the higher was apparent molecular weight and hydrodynamic radius. Moreover, in order to prepare micelles not only with the pH-controlled release of drug but even with the pH-controlled disintegration of micelles themselves, we used hydrazone bond as a pH-sensitive linkage between HS and carrier.
Acknowledgement: This work was supported by Zentiva a.s. and by the Grant Agency of Academy of Sciences of the Czech Republic (grant No. IAA4050201).
References: 1. Etrych T. et al., Macromol. Biosci. 2, 43-52 (2002)
2. Ulbrich K. et al., J. Controlled. Release 87, 33-47 (2003)
3. Maeda H. et al., Crit. Rev. Ther. Drug Carrier Syst. 6, 193 (1989)
4. Patent No. CZ PV 2006-207
N. SOLOMKOa, O. BUDISHEVSKAa, S. VORONOVa
aInstitute of chemistry and chemical technologies, National university "Lvivska polytechnika", str. Bandery 12, Lviv, 79013, Ukraine
budish/polynet.lviv.ua (solomko_nadia/rambler.ru)
One of the most important problems of chemistry of medical-biological polymers is creation of macromolecular systems, which are capable to discharge physiological active substances, which they contain. The change of external conditions as pH, temperature, or chemical conditions is the cause of this discharge.
The hydrogels were obtained with use of natural polymer chitosan - poly-b-1,4-(2-deoxy-2-amine-D-glucose) and poly-(N-vinyl-2-pyrrolidone). Chitosan - polysaccharide, which is insoluble in water and organic solvents, but soluble in acidic aqueous solutions - acetic, lactic, citric and so on. Amine groups of chitosan main-chain links is protonated and ionized in acidic solutions. Macromolecule of chitosan is polyelectrolyte with positively charged ammonium groups situated along chain. This ammonium groups are cations in chitosan-acids salts.
Tert-butylperoxymethylmaleate (TBPMM) was used for dissolving of chitosan in aqueous environment. TBPMM formed salt with amine groups of chitosan main-chain links.
Hydrogels were obtained by radical copolymerization chitosan and N-vinyl-2-pyrrolidone with initiated radicals, which are formed by primary-tertiary peroxide groups thermo-decomposition.
It was shown that degradation of TBPMM is accompanied by fragmentation of tert-butoxyl and maleic-methoxyl radicals and propanone, 2-methyl-2-propanol, methanol, carbonic dioxide and remainder of unsaturated acid was formed. The remains of unsaturated acid and TBPMM are cross-linking agents of gel formation.
It was shown, that swelling of gels depends on pH, quantity of TBPMM in reactive mixture and deacetylation degree of chitosan.
A. Strachotaa, F. RIBOTb, J. PLEŠTILa, J. BRUSa, L. MatĚjkaa
a Department of Polymer Networks and Mechanical Properties, Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
b Institute Laboratoire de Chimie de la Matière Condensée, UMR CNRS 7574, Tour 54, 5e étage, Université Pierre et Marie Curie, 4 Place Jussieu, F‑75252 Paris Cedex 05, France
strachota/imc.cas.cz
The preparation of new, organic-inorganic epoxy networks containing the stannoxane cluster dication [(nBuSn)12O14(OH)6]2+ (Fig. 1) will be presented. The cluster dications were incorporated into polypropyleneoxide diamine - DGEBA networks through ionic bonds.
The Stannoxane clusters show a strong tendency to aggregation, which determines the mechanical properties of the nanocomposite networks prepared. The aggregation behavior was investigated by mechanical tests as well as by wide angle X-ray scattering (WAXS) and small angle X-ray scattering (SAXS) experiments.
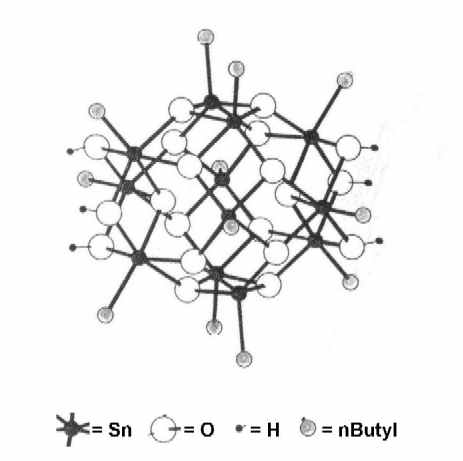
Fig.1: The Stannoxane cluster used in this work
B. STRACHOTOVÁa, L. MATĚJKAa, M. ŠLOUFa, A.STRACHOTAa, J.PLEŠTILa,
a Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic (beata/imc.cas.cz)
Super porous and thermo-responsive organic-inorganic (O-I) hydrogels were prepared from N-isopropylacrylamide (NIPA), N,N'-methylenebisacrylamide (BAA) and tetramethoxysilane (TMOS) by simultaneous radical polymerization and hydrolytic polycondensation of TMOS in aqueous solution.
The formed hydrogels display not only a rapid deswelling, but in contrast to most fast responsive PNIPA gels described in the literature, they show also a very fast reswelling. Moreover, the rigid inorganic domains stabilize the pore structure of the O-I hydrogels and improve their mechanical properties.
The exact synthesis procedure has a decisive influence on the final gel properties and was typically carried out in two steps: The first step proceeded in a homogeneous solution, while during the second, the temperature was lowered so, that ice crystals formed and served as pore templates.
A. MINAŘÍK, L. LAPČÍK Sr., P. URBAN, A. MRÁČEK, M. HRUBÁ
Institute of Physics and Material Engineering, Faculty of Technology, Tomáš Baťa University in Zlín, Nad Stráněmi 4511, 760 05 Zlín, Czech Republic, (minarik/ft.utb.cz www.ft.utb.cz/czech/UFMI )
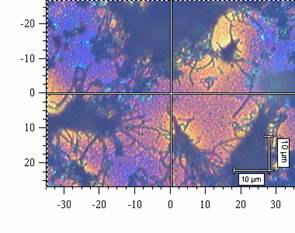
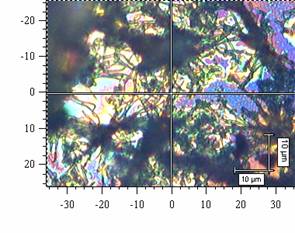
There was studied a process of self-organization of mineral particles in gel-like phases of cellulosic derivatives during solvent (water) evaporation. As an inorganic compounds were chosen: AgX (X = Cl, Br, I), ZnO, Fe2O3, and the other particular systems. It has been found that the molecular weight and the internal viscosity of the gel strongly influence the process, as well as the temperature and starting conditions inside and outside of the system. There was observed that the self-organization of mineral particles is leading to two types of aggregates, at least. First of them with the strictly simple structure (cubic type) and the second with a hexagonal one. The second one has been organized to high ordered space structures (see figure 1). The products of the processes were examined by optical microscopy, electron microscopy, termogravimetry and the image analysis. A suitable computer software was applied to the image analysis in the studied cases.
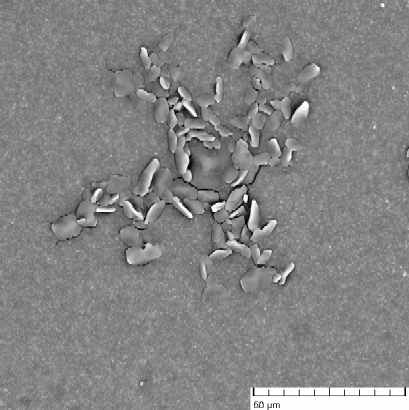
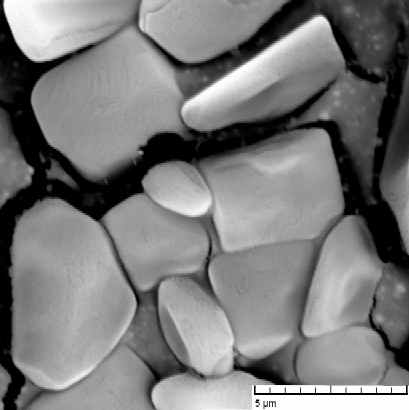
Figure 1: Aggregates of AgCl in gel phases of HEC after solvent evaporation: (a) an organized structure of a higher order, (b) typical cubic particular system. Figures have been obtained from scanning electron microscopy.
Acknowledgements: The authors would like to express their gratitude for financing of this research by Ministry of Education, Youth and Physical Training of Czech Republic (Grant VZ MSM7088352101).
N. GHAOUARb, A. GHARBIa
aLaboratoire de Physique de la Matière Molle, Faculté des Sciences de Tunis, Campus Universitaire, 2092, Tunisia.
bInstitut Nationale des Sciences Appliquées et Technologie, INSAT, 2080, Tunis, Tunisia
The system of interest is a mixture of two ramified polymers network. From a topological point of view, a ramified polymer is a network made of connected flexible long chains of the same polymerization degree, with no loops. We suppose that the connected chains are chemically identical. Concerning these systems, most of works have focused on the investigation of the configurational properties associated with star-polymers and linear network. In the previous works, Ghaouar et al [1-4] are examined in details this system and determined all topological, scattering and critical properties for neutral and charged systems.
In fact, we will propose here to studied the effects of the initially density fluctuation on the structure and the kinetics of the microphase separation. For this, we use the de Genne's theory proposed for interpenetrating networks. Indeed, he considers a mixture of two chemically different polymers cross-linked in the one phase region. Because the presence of the cross-linked, there is then a competition between the monomer-monomer repulsion and the elastic forces that resist the phase separation. There results a microphase separation, where finite domains rich in each of the components are present. The study of these systems is renewed, when Brider and Bauer test the de Gennes prediction about the microphase separation by small-angle neutron scattering experiments for a mixture of polymers namely polystyrene and poly(vinyl methyl ether). This mixture is miscible at room temperature and may be cross-linked in a controlled way by irradiation. They found that there is an acceptable agreement between the experimental results and theory, but they declare a notable exception in the zero-angle scattering. The latter was assumed to be vanishing in the calculations, whereas it was definitely non-vanishing and rather large in the experiments. Thus, this discordance is originated that de Gennes consider that no fluctuations are present in the initial mixture. Indeed, when the system is cross-linked these fluctuations are frozen-in. So, we assume that two temperatures are present in the system of interest and not only one as in the de Gennes theory. These are the initial temperature when the cross linking process is taking place and the final one at which the system is quenched after cross-linking. This fact is natural for our considered system, because from its definition a ramified polymer is constituted by connecting chains at some reticulation point.
Indeed, we first applied the de Gennes theory for the ramified polymer network and we present respectively our originate results using the initially fluctuations effects by introducing a cut-off length in the structure factor expression. Secondly, we study the kinetics of the microphase separation. More exactly, we aim at a quantitative investigation of the topology influence on demixing transition. The kinetics will be studied through the growth (or relaxation) rate of the composition fluctuation. This rate can be expressed in terms of the static structure factor and the Onsager transport coefficient. Thirdly, we are interested to the influence of the presence of solvent on the kinetics of the microphase separation where the initial density fluctuations are present.
Reference
[1]: N. Ghaouar, M. Benhamou, A. Derouiche, A. Gharbi, Eur. Phys. J. E 16 (2005) 409.
[2]: M. Benhamou, N. Ghaouar, A. Gharbi, M. Benmouna, Condensed Matter Physics, 1(37) (2004) 179.
[3]: N. Ghaouar, M. Benhamou, A. Gharbi, Physica A 359 (2006) 267.
[4]: N. Ghaouar, A. Gharbi, Physica A, Accepted for publication.
[5]: R.M. Briber, B.J. Bauer, Macromolecules 21 (1988) 3296.
A. BODROVA, I. POTEMKIN
Lomonosov Moscow State University, Physical Faculty, 119992, Moscow, Russia.
There are many of experimental publications on the investigation of the polymer systems with counterions differing in size. On the other hand, the effect of the size of counterions was not studied theoretically yet. In this paper the influence of the size of counterions on the collapse of polyelectrolyte gel is studied. The possibility of ion pair formation between charged groups of the gel and counterions is also taken into account. The model of monomer units, counterions and ion pairs as hard spheres of different radius is considered. Volume interactions between monomer units and counterions, counterions and ion pairs, monomer units and ion pairs, counterions themselves and ion pairs themselves are taken into account. The free energy of volume interactions is calculated using the virial expansion. The second and third virial coefficients are calculated. The collapse has been investigated for different potentials of interaction between the particles. The influence of the dielectric constant of the system on the collapse has also been investigated.
It was found, that the volume interactions between counterions are not important for the behavior of the weakly charged polyelectrolyte gel. If the dielectric constant is big, the transition from swollen into collapsed state is discontinuous for the network with small counterions. When the counterions become larger, the transition becomes continuous. If the monomer units-counterions and counterions-counterions interactions are repulsive, the increase of the size of counterions leads to the shift of the transition towards poorer quality of the solvent and to increasing of the swelling coefficient. If the monomer units-counterions and counterions-counterions interactions are attractive, the swelling coefficient decreases with increasing of the counterion's size. If the dielectric constant is small, all point counterions form ion pairs, and large quantity of large counterions remains free. Therefore the gel with point counterions behave like neutral gel in this case, and the behavior of the gel with large counterions does not depend on the dielectric constant.
I. A. ANSARI, N. CLARKE, L. R. HUTCHINGS, A. PILLAY-NARRAINEN, R. L. THOMPSON
UIC in Nanotechnology, Department of Chemistry, University of Durham, Durham, DH1 3LE, United Kingdom, Email: i.a.ansari/durham.ac.uk
We have synthesized a series of linear deuterio-polystyrene (dPS) chains from fluorocarbon (FC) functionalised dendritic initiators (Fig. 1). This approach enables us to add relatively large quantities of surface-active fluorocarbon groups onto the end of a polymer that is compatible with a bulk polymer. We are investigating the effect on self-segregation of polymers carrying multiple end-functionality by various techniques.
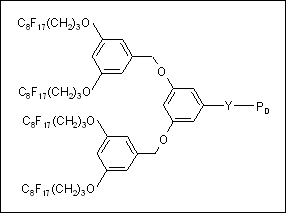
Fig. 1: Sketch of 4xFC dendron functionalised polymer chain with PD as a linear dPS chain
We have studied the adsorption, diffusion and aggregation properties of these materials in blended films with unfunctionalised polystyrene. Nuclear reaction analysis (NRA) showed that the functionalised polymers spontaneously segregate to the film surface within a few seconds of the spin-coating process. This indicates that these materials have an appreciable surface – activity, even in solutions. Surface excess concentrations measured approach closely the value expected for saturated monolayer from which the homopolymer is largely excluded.
Contact angle measurements using both dodecane and water indicates a substantial (~50%) reduction in surface energy as saturation of the surface is approached.
Small Angle Neutron Scattering (SANS) experiments helped in understanding the aggregation of these materials in bulk while Neutron Reflectivity (NR) showed the possibility of multilayer absorption. The results obtained by SANS compliment our findings by NRA that these materials adsorb extremely strongly to film surfaces. The evidence from NR data for a possible multilayer absorption was confirmed by Transmission Electron Microscopy (TEM).
H.-P. HSU, W. PAUL, K. BINDER
Institut für Physik, Johannes Gutenberg-Universität Mainz
Staudinger Weg 7, D-55099 Mainz, Germany
Homopolymer and alternating copolymer bottle-brushes with straight rigid backbones are studied in three dimensions. They are described by self-avoiding random walks on a simple cubic lattice with and without nearest neighbour attractions between like monomers in a poor and a good solvent, respectively. For the simulations, we employ a very efficient chain growth algorithm with resampling, PERM, modified to allow simultaneous growth of all side chains. The scaling behaviour of bottle-brushes with various grafting densities is compared with theoretical predictions. With increasing repulsive interactions between unlike monomers, we verify the existence of phase separation due to the incompatibility between chemically different side chains. We also show that no true phase transition occurs.
R. Ben Arfi*, M. Brogly †, J. Schultz
Institut de Chimie des Surfaces et Interfaces (ICSI)-CNRS
UPR9069
B.P. 2488-15 rue Jean Starcky-68075 Mulhouse Cedex (Tél.: (33) 3
89 60 87 68
Fax : (33) 3 89 60 87 99)
E-mail : *rim.ben-arfi/uha.fr
†m.brogly/uha.fr
WE
Self-assembled monolayers (SAMs) offer numerous technological applications in nano-fabrication of electronic devices and biomaterials, adhesion, thin-film lubrication, and corrosion protection.
The understanding of mechanisms governing the growth, the structure and the conformation of SAMs is determinant for an optimal use in any application.
Alkylthiols were adsorbed from solution onto different metals (gold, copper and zinc) in order to reveal the influence of the substrate in defining and controlling the organisation and reactivity of self-assembled monolayer films.
A variety of analytical techniques were used to characterize the different substrates: wettability, ellipsometry, atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS) and the polarization modulation infrared reflection absorption spectroscopy (PM-IRRAS).
Results have shown that alkanethiol molecules chemisorb spontaneously from solution on metallic surfaces through the thiol headgroup. Adsorption onto three metals generates monolayer films. Observations suggest that the structure of films is controlled by varying the concentration of the solution and the assembly time. The packing density, the organization and the conformation of the molecular chain depend on the nature of the metal substrate and on the roughness of the surface.
All results presented in this work demonstrate the interest of multi-techniques and multi-scales approach in the characterization of ultra-thin organic films.
J. PROKEŠa, I. ŠEDĚNKOVÁb, M. TRCHOVÁb, J. STEJSKALb
aCharles University Prague, Faculty of Mathematics and Physics, 121 16 Prague 2, Czech Republic
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky sq. 2, CZ-162 06 Praha 6, Czech Republic (sedenko/imc.cas.cz)
Polyaniline (PANI) films were produced at room temperature from aniline hydrochloride oxidized with ammonium peroxydisulfate on glass surfaces immersed in the reaction mixture.1 In-situ produced films have a typical thickness of the order of hundreds of nanometres. Ageing process was done in the thermostat chamber having a low humidity. Temperature of samples was elevated from 25 °C during about 1 h to the ageing temperature. DC resistivity was measured by the four-point method in van der Pauw configuration.
A notable change in the character of the time dependence of resistivity has been observed when temperature of ageing passed 85 °C. The ageing was much faster above this limit. We believe that this effect is caused by structural transition at about 85 °C. This statement is supported by the FTIR spectroscopic measurements done on doped as well as undoped samples. The temperature dependence of the ratio of the integral intensities of the bands associated with the benzenoid and quinonoid units shows a maximum close to 80 °C (Fig. 1). The FTIR spectral variation may be explained by conformational transition of the polymer chain. The fact that a similar transition has been found with undoped samples indicates that this feature is inherent property of PANI, and not the influence of the dopant acid.
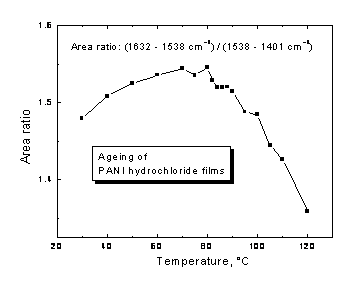
Fig. 1. The changes in the molecular structure reflected in FTIR spectra
1. J. Stejskal, I. Sapurina, J. Prokeš, J. Zemek, Synth. Met. 105 (1999) 195.
Acknowledgment. This work is a part of the research plan MSM 0021620834 that is financed by the Ministry of Education, Youth, and Sports of the Czech Republic. The authors thank the Grant Agency of the Academy of Sciences of the Czech Republic (A4050313 and A400500504).
S.V. SOLOSINa, I. ŠEDĚNKOVÁa, M. TRCHOVÁa, J. STEJSKALa
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky sq. 2, CZ-162 06 Prague 6, Czech Republic (solosin/imc.cas.cz)
Polyaniline (PANI) films were prepared in situ on silicon windows during the oxidation of aniline with ammonium peroxydisulfate in aqueous solutions of strong (sulfuric) or weak (acetic) acids. It has been shown earlier that the courses of polymerization, followed by temperature and acidity changes, are different.1 In the solutions of sulfuric acid, a granular PANI was produced, in the solutions of acetic acid, PANI nanotubes were obtained (Fig. 1). The molecular structure of the films produced on silicon windows under different conditions (in solutions of sulfuric, succinic, and acetic acid or without any acid) has been studied by FTIR and Raman spectroscopies. The differences between the spectra reflect the variations of molecular structure, which leads to the different morphology of PANI. The products of polymerization in presence of weak acid or in the absence of any acid are markedly sulfonated and they contain phenazine units.
The supramolecular structure of the final films is connected with cross-linking and formation of phenazine-like structures in PANI. The internal protonation, leading to ionic interaction between sulfonate groups and protonated imine sites of PANI chain, together with hydrogen bonding, help to stabilize the nanotubular structure.
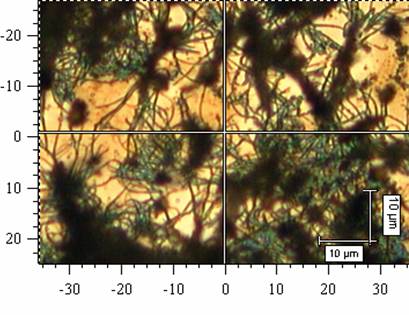
Fig. 1. The evolution of polyaniline nanotubes
1. Konyushenko E. N., Stejskal J., Šeděnková I., Trchová M., Sapurina I., Cieslar M., Prokeš J.: Polyaniline Nanotubes: the Conditions of Formation, Polym. Int. 55 (2005) 31.
I. ŠEDĚNKOVÁa, Š. GREGOROVÁb, S.V. SOLOSINa, M. TRCHOVÁa, J. STEJSKALa
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky sq. 2, 162 06 Prague 6, Czech Republic (sedenko/imc.cas.cz)
bGymnázium Nad Alejí, Nad Alejí 1952, 162 06 Prague 6, Czech Republic
Polyaniline (PANI) films1 were prepared in situ on silicon windows during the oxidation of aniline with ammonium peroxydisulfate in aqueous solutions of strong (sulfuric) or weak (acetic, succinic) acids or without any acid. In the solutions of sulfuric acid, a granular PANI was produced, in the solutions of weak acids or without any acid; PANI nanotubes were obtained (Fig. 1, left). The thermal stability and structural variation of the films produced on silicon windows under different conditions was studied by FTIR and Raman spectroscopies. The results indicate that spectral changes correspond to deprotonation, oxidation and thermal transition due to the chemical cross-linking reactions among PANI molecules. PANI bases are more stable than the salt forms after thermal treatment at 80 °C during three months.
 |
 |
| FIG. 1. Polyaniline films prepared in 0.4 M succinic acid before (left) and after ageing (right) | |
The films of PANI salts loose their protonating acid. The films obtained in water or in the presence of acetic acid are more stable than those prepared in the solutions of sulfuric or succinic acids.
1. M. Trchová, I. Šeděnková, E.N. Konyushenko, J. Stejskal, P. Holler, G. Ćirić-Marjanović, J. Phys. Chem. B 2006, in press.
N.V. BLINOVA, M. TRCHOVÁ, J. STEJSKAL
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky sq. 2, CZ-162 06 Prague 6, Czech Republic (blinova/imc.cas.cz)
Polyaniline (PANI), a conducting polymer, is typically prepared by the oxidation of aniline hydrochloride with ammonium peroxydisulfate in acidic aqueous medium. Protonated PANI is obtained as a green precipitate, ammonium sulfate and sulfuric acid are by-products. The conductivity of PANI is1 4 S cm–1.
Let us design the experiment, in which solutions of reactants are separated by a semipermeable cellulose membrane. The polymerization of aniline starts at the membrane, which converts to a self-assembled PANI membrane. The molecules of a reductant (aniline) and an oxidant (peroxydisulfate) can then exchange electrons through the conducting membrane, and thus to undergo redox reaction2, without any mutual contact (Fig. 1). Polyaniline is therefore produced only on one side of the membrane.
FIG. 1. When both a monomer and oxidant are separated with a
PANI membrane, PANI is produced only on left side of the
membrane.

The electroneutrality of the system is maintained by the synchronous transport of protons through the PANI membrane, which is also proton conducting (Fig. 1). Protons are generated from the hydrogens, which are abstracted from aniline molecules; they constitute sulfuric acid. Mechanism of redox reaction on the PANI membrane has been analysed with the help of FTIR spectra.
1. Stejskal, J.; Gilbert, R.G. Pure Appl. Chem. 2002, 74, 857.
2. Kocherginsky, N.M.; Lei, W.; Wang, Z. J. Phys. Chem. B 2005, 109, 4010.
O.V. Khutoryanskaya, A.C. Williams, V.V. Khutoryanskiy*
School of Pharmacy, University of Reading, Whiteknights, PO Box 224, Reading RG6 6AD Berkshire, United Kingdom. E-mail: v.khutoryanskiy/reading.ac.uk
Interaction between poly(acrylic acid) and methylcellulose in aqueous solutions leads to the formation of hydrophobic interpolymer complexes (IPC), stabilised by hydrogen bonds. In this communication we will report three different approaches for preparing polymeric materials based on poly(acrylic acid) and methylcellulose. The first approach is based on casting solution mixtures of the two polymers without pH adjustment and subsequent drying in air, which results in bulk polymeric film formation. The second approach utilises mixing polymer solutions under acidic conditions leading to the formation of interpolymer complexes in the form of a dispersion, which gradually precipitates. In the third approach, thin multilayered IPC films can be prepared via layer-by-layer sequential adsorption of poly(acrylic acid) and methylcellulose on the surface of glass under acidic conditions. The materials prepared have been characterised by infrared spectroscopy, differential scanning calorimetry, scanning electron microscopy and solubility/swelling property testing. The potential applications of these materials in dosage forms design will be discussed.
N.Nosova, V. Samaryk, S.Varvarenko, S.Voronov
"Lvivska Polytechnika" National University, Lviv, Ukraine, budish/polynet.lviv.ua
The control of polymer product surface properties is an issue of the day. Its solution allows to extend the fields of industrial plastic application due to combination of modified surface properties and retained bulk properties of polymer. The large number of modification techniques for these materials has been elaborated that include the step of surface peroxidation. Activation of the surfaces via their peroxidation is mainly performed, in accordance with these techniques, with utilization of hard treatment conditions (irradiation with high-energy particles, treatment with strong inorganic oxidants).
We have developed an alternative route of polymer surface peroxidation via grafting of polyperoxides containing aliphatic perester or ditertiary peroxide groups in their structure. Under this technique, grafting is initiated at the expense of partial thermolysis of peroxide groups from polyperoxide composition. Surface peroxidation is provided by peroxide groups that are not decomposed during polyperoxide grafting to polymer surface. Due to peroxide group presence on the surface it is possible to modify it in the sequel. In terms of this work, modification of peroxidized polypropylene surface has been performed via polymerization of acrylic acid, acrylamide, and p-vinylpyridine that was initiated from the surface. Furthermore, a study of radical grafting of dextran, dextran sulfate, and heparin to peroxidized surface has been performed in order to create surface layers of special properties.
Surface modification process is controlled at every stage using a technique of free surface energy determination via measurements of contact angles by two liquids and using an ellipsometry.
The studies performed have shown the possibility of polymer material surface activation (polypropylene, polyethylene terephthalate, polyethylene etc.) using polyperoxides with further modification of peroxidized surface. The experimental data obtained have allowed to formulate theoretical grounds of surface modification with polyperoxides and, on this basis, to forecast the structure of peroxidized layer grafted covalently to polymer surface.
A.K. BERKOVITCH, N.S. MELIK-NUBAROV
Polymer division, Chemistry department, M.V. Lomonosov Moscow State University, Leninskie gory 1-3, Moscow, Russia
annber/yandex.ru
Investigation of the interactions of phospholipids and synthetic polymers are important for the understanding of the structure and functioning of biological macromolecules in membranes. Natural phosphatidylcholine (PC) has zwitter-ionic structure and it does not bear any charge under neutral conditions. But in the slightly acidic medium phosphate groups of a portion of PC molecules become protonated and the lipid acquires net positive charge. So, the net charge of lipid bilayer becomes positive as pH decreases, benefiting electrostatic interaction with polyanion.
It was shown that different polyacids adsorb on liposomes composed of egg yolk lecithin under slightly acidic conditions (pH<5). Interaction of polyacids with vesicles under slightly acidic conditions resulted in the formation of hydrophilic pores in liposome membranes, permeable for the charged low molecular compounds. The ability of polymers to permeabilize bilayer was shown to depend upon their structure. Thus, the polymer flexibility, hydrophobicity and acidic strength were hypothesized to be the main determinants of permeabilizing activity.
However, the polymer effect on the membrane permeability could not be explained only by the formation of electrostatic bonds with the vesicle surface. It has been demonstrated that an addition of dipole modifier phloretin resulted in the substantial decrease in the polymer effect on the membrane permeability. This fact suggests that dipole potential of the bilayer is an important factor favoring a deepening of the polymer into the hydrophobic core of the membrane and thus formation of pores or channels in the bilayer.
The work was supported by Russian Foundation for Basic Research (grant №06-03-32403)
M. Retsch, A. WALTHER, A. Müller
Macromolecular Chemistry, University of Bayreuth, D-95447 Bayreuth, Germany (markus.retsch/uni-bayreuth.de, andreas.walther/uni-bayreuth.de, axel.mueller/uni-bayreuth.de, www.mcii.de )
The functionalization of surfaces has attracted more and more interest throughout the last years. In this work we present various ways to synthesize poly(acrylic acid) brushes onto gold surfaces using the “grafting from” approach via controlled radical polymerization. As a first step a self-assembled monolayer (SAM) of the initiator for the controlled radical polymerization was deposited on the surface. Then ATRP of tert-butyl acrylate was conducted in presence of a sacrificial initiator to achieve films of controlled layer thickness. The molecular weights of the polymers in solution were determined by gel permeation chromatography and the films before and after deprotection of the tert-butyl groups were characterized by ellipsometry, atomic force microscopy, water contact angle, surface plasmon resonance (SPR) and infrared spectroscopy (grazing angle FTIR). These functionalized surfaces were used to study the interaction between silsequioxaen nanoparticles and poly(acrylic acid) brushes. These interactions were followed by SPR.
P. DALLASa*, D. NIARCHOSa, D. PETRIDISa
aNCSR,“Demokritos”,Institute of Materials Science, Athens, 15310, Greece.
*E-mail: dallas/ims.demokritos.gr
We present a one step synthetic route of polypyrrole architectures in submicron level and in situ formation of silver nanoparticles (8 nm) through an interphasial water/chloroform system.The synthetic root involves the polymerization of pyrrole in a water/chloroform interphase and in situ formation of silver nanoparticles through a redox reaction of Ag(I) with pyrrole monomers.Ag(I) or Fe(III) used as oxidizing agents were dissolved in H2O and the mixture was added in a chloroform solution in which pyrrole monomer has been previously added.The surfactants added in the organic media were dodecyltrimethylammonium bromide and dodecylsulfate sodium salt.The progress of the polymerization was studied with UV spectroscopy, which reveals the polaron nature of the conjugated polymer.Since the polymerization of pyrrole proceeds through the formation of ions the oligomers are observed in the aqueous phase.Peaks corresponding to π-π* transitions or polaron excitations where observed, appearing in different wavelengths according to the addition or not of surfactants.The morphology and the crystallinity of the samples varied according to the added surfactant.SEM images show that the polymer consists from parts with a diameter range 200-300 nm that coincide to form a network extended to several microns.These parts have almost uniform size distribution and are bound together.Further characterization was performed with TEM,EDS analysis,FT-IR,TGA/DTA,conductivity measurements.
a)
b)
c)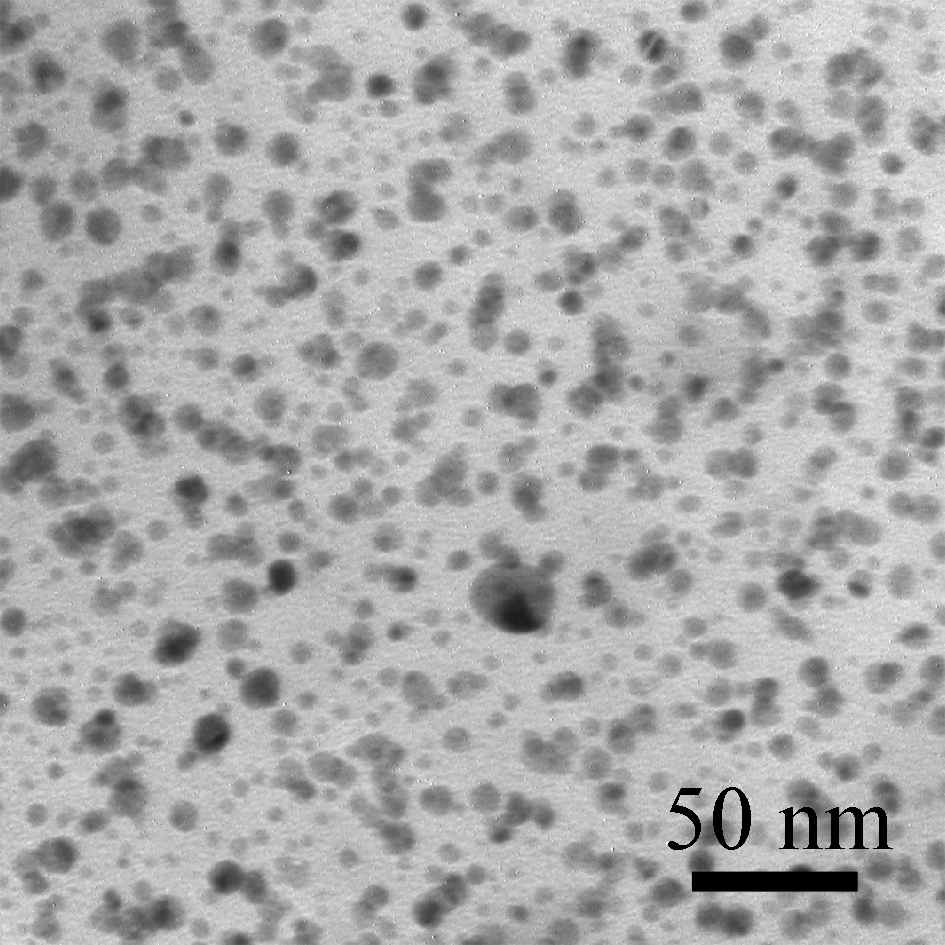
| FIG. 1 a)Ppy synthesized with dodecyltrimethylammonium bromide | b)Ppy synthesized in the presence of dodecylsulfate sodium salt | c)TEM micrograph of the silver nanoparticles embedded in the polypyrrole matrix. |
L.M. YARYSHEVA*, E.G.RUKHLYA**, O.V. ARZHAKOVA*, A.A. DOLGOVA*, A.L.VOLYNSKII*, N.F. BAKEEV**
*Chemistry Department, Moscow State University, Leninskie gory, Moscow, 119992 Russia
**Institute of Synthetic Polymer Materials, Russian Academy of Sciences, ul. Profsoyuznaya 70, Moscow, 117393 Russia
Deformation of amorphous glassy and semicrystalline polymers in the presence of adsorptionally active liquid environments proceeds via the mechanism of solvent or wet crazing. This process is accompanied by polymer dispersion into fine aggregates of oriented macromolecules (fibrils), which are separated by microvoids. The dimensions of craze fibrils and microvoids do not exceed 20 nm. Highly dispersed and porous polymer materials prepared via wet crazing can serve as suitable matrices or substrates for the preparation of nanocomposites and polymer blends. The advantages of the proposed approach are the following: as this method does not require any thermodynamic compatibility between components, one can use quite different polymers and additives and prepare wide range of polymer blends.
In this work, we studied the blends based on wet-crazed polymers prepared by the mechanism of classical crazing (PET) and delocalized wet crazing (HDPE). As second component, we used such polymers as PMMA, PS, PBMA, poly(ethylene glycol), polyaniline, polyacetylene, etc. Mechanism of wet crazing and conditions of tensile drawing of polymers in liquid environments allow one to monitor structure, composition, and properties of as-prepared blends. The content of incorporated component in polymer blends can achieve 40-50%. Structure of the blends based on wet-crazed polymers is characterized by microphase separation of constituent components into two continuous phases; thus, it can be classified as semiinterpenetrating polymer networks. Let us mention that, in this case, one component or both components possess molecular orientation. As the as-prepared blends are based on different polymers, their final characteristics combine the advantages of both components. For example, electric conductivity of PET-polyaniline and HDPE-polyaniline blends is comparable to that of electroconducting component (polyaniline), whereas the final mechanical properties of the blends are controlled by the mechanical properties of robust polymer matrices. The blends of PET and HDPE with hydrophilic polymers are characterized by improved wettability. Hence, porous polymer matrices prepared via wet crazing can serve as efficient matrices for the preparation of polymer blends with a high level of mutual dispersion between components, and the final materials offer new fascinating properties.
This work was supported by the Russian Foundation for Basic Research (project no. 06-03-32452).
S.V. Moiseeva**, D.A. Panchuk*, A.V .Bol'shakova*, L.M. Yarysheva*, A.I. Dement'ev**, A.L. Volynskii*, N.F. Bakeev*
*Chemistry Department, Moscow State University, Leninskie gory, Moscow, 119992 Russia
** Chemistry Department, Moscow Pedagogical State University, Nesvizhskiy per., 3, Moscow, 119882 Russia
Polymer films with a thin rigid coating (metals, metal oxides) are widely used as flexible mirrors, oxygen-insulating, sensor, magnetic, and electroconducting materials. However, tensile drawing or thermal treatment of such films can lead to the fracture of the coating and can disturb its integrity. In this connection, it seems important to gain a deeper insight into the character and mechanism of fracture of a rigid coating during plastic deformation of polymer support and to investigate into the effect of stretching conditions on this process.
Uniaxial stretching of the films based on amorphous PET with a thin metallic coating (aluminum, gold, platinum) is accompanied by the breakdown or disintegration of the coating into fractured fragments as asymmetric ribbons, which are primarily directed perpendicular to the direction of tensile drawing, and polymer surface layer acquires a regular relief.
Examination of the structure of the surface layer of the PET sample with aluminum coating before and after the elimination of metallic layer shows that the fractured fragments are primarily composed of polymer, and only their top is covered by the metallic layer. The formed surface relief is provided by the fracture of the coating and by inhomogeneous character of tensile drawing of the surface layer at the regions connected with metal and located between the fractured fragments.
The dependences of the dimensions of fractured fragments on the conditions of tensile drawing and their width distributions are analyzed. Fracture of the coating during stretching of PET is found to take place in the region of the formed neck, and predominant mechanism of fracture is disintegration of the fractured fragments into two equal parts.
Fragmentation of the coating is studied for the samples with different thickness of the coating (7-27 nm). With increasing the thickness of the coating, the general pattern of fragmentation remains unchanged; however, the dimensions of the fractured fragments are shown to increase. Taking into account the dependence of the width of the fractured fragments on the coating thickness, mechanical properties of metals (gold and platinum) in thin layers are analyzed.
This work is supported by the Russian Foundation for Basic Research (project no. 06-03-32452, 05-03-32538).
b Intense Pulsed Neutron Source, Argonne National Laboratory, Argonne, IL 60439, USA (jlal/anl.gov)
Nanostructured polymers, a substantial part of nanostructured materials and surfaces, provide attractive avenues for the development of nanotechnologies, optically active materials, biomaterials, membranes, battery components, electronics and photonics.
“Bottom-up” nanofabrication uses directed assembling of molecular-scale entities to create larger scale ones with engineering properties. Self-assembly of block copolymers or functionalized nanostructures can generate a rich variety of nanomorphologies (lamellar, cylindrical, gyroid, various cubic etc.)
Phenomena associated with the order-disorder transition (ODT), microdomain morphology and phase behaviour of two block copolymer systems have been studied by small-angle X-ray scattering (SAXS), small-angle neutron scattering (SANS) and transmision electron microscopy (TEM).
1) The microdomain morphology and phase behaviour in copolymer blends of A-b-B/A-b-C i.e. with one block in common (deuterated PS-b-PMMA / deuterated PS-b-PI) were investigated by SAXS, SANS and TEM. The studied almost symmetric copolymers differ essentially in microdomain morphology. One of them is in disordered microdomain state, while the other displays lamellar morphology at ordinary temperatures. Self-assembled structures in blends were investigated as a function of concentration of the added microphase-separated copolymer and temperature.
2) Self-assembled structures in binary mixtures of A-b-B/A (deuterated PS-b-PMMA/PS homopolymer) were studied as a function of molecular weight and concentration of the added polymer. A criterion for “wet and dry brush” behaviour respecting different solubilization of the homopolymer in microdomain morphology has been applied to explain the changes in microdomain morphology.
J. LOKAJa, L. POLÁKOVÁa, P. HOLLERa, P. ŠTĚPÁNEKa, O. DIATb
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
bStructure et Propriétés d´Architectures Moléculaires, UMR 5819 (CEA-CNRS-UJF), DRFMC/SPrAM, CEA-Grenoble, 38054 Grenoble cedex 9
A number of new cationic block copolymers with trimethylammonium groups were synthesized and characterized. The synthesis employed a nitroxide-mediated radical polymerization technique for the preparation of block copolymers containing functional 2-(dimethylamino)ethyl methacrylate (DAMA) units which were subsequently quaternized with methyl iodide.
Generally, radical homopolymerization or copolymerization of styrene (S) with acrylonitrile (AN) or N-butylmaleimide (BuMI) in the presence of 2,2,6,6-tetramethylpiperidin-1-yloxy radical (TEMPO) was performed and quasiliving polymers with reversibly bound TEMPO end groups were obtained. The quasiliving polymers served as macroinitiators in chain extension with DAMA and, in the case of S-AN and S-BuMI copolymers, also with styrene. In TEMPO-mediated radical copolymerization of DAMA with styrene, the DAMA units were directly incorporated in the macroinitiator; then, chain extension with styrene was conducted. Using TEMPO-terminated poly(styrene-co-acrylonitrile)-block-polystyrene or poly(styrene-co-N-butylmaleimide)-block-polystyrene diblock copolymers as macro-initiators in the polymerization of DAMA, triblock copolymers containing outer poly(DAMA) blocks were obtained.
Quaternization of DAMA units in the copolymers with methyl iodide afforded corresponding block polyelectrolytes. The latter formed hydrophilic films by casting dimethylformamide solutions and evaporation of the solvent.
The authors gratefully acknowledge support by the Grant Agency of the Czech Republic (SON/03/E001) within the EUROCORES Programme SONS of the European Science Foundation, which is also supported by the European Commission, Sixth Framework Programme.
J. LOKAJa, L. POLÁKOVÁa, P. HOLLERa, P. ŠTĚPÁNEKa, O. DIATb
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
bStructure et Propriétés d´Architectures Moléculaires, UMR 5819 (CEA-CNRS-UJF), DRFMC/SPrAM, CEA-Grenoble, 38054 Grenoble cedex 9
Radical polymerization in the presence of nitroxide produces quasiliving nitroxide-capped polymers with narrow molecular weight distributions. The nitroxide-terminated polymers can be employed as macroinitiators in chain extension with an appropriate monomer or comonomers under formation of corresponding polymer blocks.
This study is focused on the preparation of amphiphilic block copolymers by chain extension using 2,2,6,6-tetramethylpiperidin-1-yloxy radical (TEMPO) as the capping agent. Polystyrene, styrene-acrylonitrile or styrene-N-butylmaleimide copolymers containing terminal TEMPO groups were synthesized to initiate polymerization of 2-(dimethylamino)ethyl methacrylate (DAMA). Diblock copolymers comprising both hydrophobic polymer precursor and hydrophilic poly(DAMA) blocks were obtained. With polystyrene macroinitiator, a limited amount of DAMA participated in the polymerization. The content of incorporated DAMA units and, consequently, molecular weights of the resulting diblock copolymers did not change significantly with the reaction time. Evidently, deactivation of polymer radicals containing terminal DAMA units took place in the polymerization process. The limited growth of DAMA was also observed using the other prepared macroinitiators. Chain extension of nitroxide-capped poly(styrene-co-acrylonitrile)-block-polystyrene or poly(styrene-co-N-butylmaleimide)-block-poly-styrene with DAMA afforded amphiphilic triblock copolymers.
Nanostructured films were prepared by casting chloroform solutions of the prepared functional block copolymers and subsequent evaporation of the solvent.
The authors gratefully acknowledge support by the Grant Agency of the Czech Republic (SON/03/E001) within the EUROCORES Programme SONS of the European Science Foundation, which is also supported by the European Commission, Sixth Framework Programme.
D. GROMADZKIa, R. MAKUŠKAa, J. LOKAJb, M. JANATAb, M. NETOPILIKb, P. ŠTĚPÁNEKb
aDepartment of Polymer Chemistry, Vilnius University, Naugarduko 24, LT-03225 Vilnius, Lithuania(d_grom/interia.pl)
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
Block and graft copolymers containing ionic segments are among the most important classes of macromolecules1. For the synthesis of such complex materials with well-defined polymer architecture the controlled/living polymerizations are generally employed.
In this work we used photoiniferter quasi-living radical polymerization to design functional ion-containing graft copolymers2,3,4. A multifunctional polystyrene macroinitiator (PS-DC) carrying 18 mol % of N,N-diethyldithiocarbamate (DC) pendant groups was synthesized by conventional radical copolymerization of styrene and vinylbenzyl N,N-diethyldithiocarbamate or by TEMPO-mediated radical polymerization of styrene and chloromethylstyrene (CMS) followed by dithiocarbamylation of CMS units.
The PS-DC maroinitiator was UV-irradiated in the presence of selected derivatives of (meth)acrylic acid, such as 2-tetrahydropyranyl methacrylate (THPMA), tert-butyl methacrylate (tBuMA) and tert-butyl acrylate (tBuA) and cationic monomer 2-(dimethylamino)ethyl methacrylate (DMAEMA) in bulk and in solution.
Graft copolymers with different length of THPMA, tBuMA, tBuA and DMAEMA segments were easily prepared by controlling the photoirradiation time. The graft copolymers were characterized by both IR and 1H NMR spectroscopy as well as by GPC.
Polystyrene-graft-poly(meth)acrylic acid amphiphilic copolymers were subsequently prepared by deprotection of the acid functionality on the tBuMA, tBuA and THPMA segments by acidic hydrolysis in refluxing dioxane.
Acknowledgement: Support by the fund from European Commission within Marie Curie Research Training Network MCRTN-SOCON, Sixth Framework Programme, is acknowledged.
References:
1. A. B. Lowe, Ch. L. McCormick, Chem. Rev. 2002, 102, 4177
2. T. Otsu, M.Yoshida, Makromol Chem, Rapid Commun 1982, 3, 127
3. T. Otsu, J Polym Sci Part A: Polym Chem 2000, 38, 2121
4. A. Šebenik, Prog Polym Sci 1998, 23, 875
V. RYUKHTINa, Z. TUZARb, P. ŠTĚPÁNEKb, K. PRANZASc
aNuclear Physics Institute, Academy of Sciences of the Czech Republic, 25 068 Rez near Prague, Czech Republic (ryukhtin/ujf.cas.cz)
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
cGKSSResearch Centre, D-21494 Geesthacht, Germany
Systems of block and graft copolymers in applicable solvents found numerous applications in many sectors of industrial technology. Ultra small-angle neutron scattering (USANS) was applied for investigations of microscopic structure of mixtures of two partially miscible solvents (a and b) and diblock copolymer (A-B) in dependence on temperature. Solvent a is a good solvent for block A, but bad solvent for block B, and vice versa for solvent b. There is a range of temperatures and concentrations where the mixtures, through remaining microscopically homogeneous, are microphase segregated into a-rich and b-rich domains separated by interfaces covered with diblock copolymer. Recent investigation of diblock copolymer where block A is polystyrene and block B is poly (ethylene-co-propylene) in dimethylformamide and deuterated cyclohexane solvents by small-angle neutron scattering (SANS) has shown that the ordered structure has hexagonal symmetry. However, the long-range periodic structure of these segregations are limited in extend, it forms µm-sized grains. These grains can neither be observed by dynamic light scattering (DLS) because relaxation time of the grains is too large nor be detected by birefringence measurements as described for polymer melts, because in this case the two used solvents are isorefractive and the resulting structure is not birefringent. Recently we have applied USANS for investigations of the grain structure in such systems.
In the following work we report on USANS experiments from poly(styrene-b-butadiene) (SB) diblock copolymer dissolved in different combinations of 12% deuterated or hydrogenated dimethylformamide (d-DMF/DMF) and rest deuterated cyclohexane (d-CX). The measurements were carried out on DCD facility at (GKSS) at λ=4.43 Å.
Almost no scattering was observed for samples with both deuterated solvents. This is explained by different content of one of the solvents in the grains in comparison with that in intergrain space. The solutions with smaller amount of diblock copolymer (10%) demonstrate higher dependence on temperature and regime of cooling. Increasing of concentration of diblock copolymer up to 20% leads to more stable microstructure of grains and a size of the grains is very weak dependent on temperature in such case.
ACKNOWLEDGEMENT
We acknowledge support by the Grant Agency of the Czech Republic (SON/03/E001) within the EUROCORES Programme SONS of the European Science Foundation, which is also supported by the European Commission, Sixth Framework Programme and by the European Commission program (RII3-CT-2003-505925).
A. SIKORSKI, P. ROMISZOWSKI
Department of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland
Sikorski/chem.uw.edu.pl
In this study we investigated the properties of star-branched polymers located in a slit. The polymer models used were rather idealized: they consisted of identical united atoms (segments), which positions were restricted to vertices of a simple cubic lattice. The force field used consisted of a simple square-well potential. The polymers were put into a slit formed by a pair of two parallel and impenetrable surfaces. The properties of the chains were determined by means of Replica Exchange Monte Carlo method combined with the multihistogram method. The influence of the temperature and the width of the slit on the polymer system were studied. The thermodynamic properties of the coil-to-globule transition at these conditions were determined and discussed.
P. ROMISZOWSKI, A. SIKORSKI
Department of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warszawa, Poland
prom/chem.uw.edu.pl
A model of a brush formed by polymeric chains was developed. The star-branched chains consisting of linear heteropolymers were constructed on a cubic lattice. Chains were terminally attached (grafted) to an impenetrable surface. The dynamic simulation of the system was performed by means of the micromodifications of local conformation basing on the Metropolis sampling algorithm. The static as well as the dynamic properties of the system was investigated for varying density of grafted chains, the temperature of the system and the specific interactions between the polymer segments. The influence of the grafting density, the chain length, as well as the sequence of polymer segments, and the temperature on the properties of the formed layer were studied and discussed. The presented model enabled one to study the structural properties of the coated surface. Also the phase transition during the annealing process of the system was described.
I. Postnova1*, E. Shumilina2, O. Duerrschmidt2, N. Ivanova1, Y. Shchipunov2
1 - Far Eastern National University
2 - Institute of Chemistry, Far East Department, Russian Academy of Sciences,Vladivostok, Russia
Soluble polyelectrolyte complexes (PECs) formed by oppositely charged polysaccharides in aqueous solutions were studied by using alginates, carrageenans and fucoidans as anionic polysaccharides and chitosan and various derivatives of hydroxyethyl cellulose (HEC) containing cationic and in some instances hydrophobic substituents, as cationic ones. The PEC formation, properties and structure were examined by a dynamic rheology, differential scanning calorimetry, NMR, optical, atomic force and scanning electron microscopy.
The PECs are formed because of the electrostatic interactions between oppositely charged functional groups of polysacharides. They result in the cross-linking of carbohydrate macromolecules. It was demonstrated by using the dynamic rheology that, as a rule, the lifetime of these linkages was shorter than the terminal relaxation time. Therefore, the PEC formation resulted in the generation of a temporal three-dimensional network from entangled macromolecules. It was shown that the scaling behaviour of such system could be described in the framework of a reptation theory, if one took into account a dependence of molecular weight of complexes on the polysaccharide concentration.
A significant increase in the cross-linking lifetime was observed for PECs of chitosan. This polysaccharide provided the formation of hard hydrogels. The jellification of its solutions depended strongly on the pH value.
For hydrophobically modified HEC derivatives, the PEC formation was depended on the location of hydrocarbon chains in the macromolecule. The interactions were weakened in a case of screened functional groups and increased when hydrocarbon chains were attached separately from charged groups.
The properties and structure of PECs by alginate, which is a natural block copolymer of two uronic acids, were regulated by its block composition. It was related to various conformations of blocks and their various flexibility-rigidity.
Iota- and kappa-carrageenans could form PECs the mechanical properties of which were changed reversibly in a certain temperature range, whereas lambda-carrageenan did not impart the same effect. The observed thermosensitivity was caused by a reversible coil-to-helix transition experienced by carrageenans even when their macromolecules were involved into the complex.
A. JAMNIKa, M. TOMŠIČa, W. KUNZb, O. GLATTERc
aUniversity of Ljubljana, Faculty of Chemistry and Chemical Technology, Aškerčeva 5, 1000 Ljubljana, Slovenia (andrej.jamnik/fkkt.uni-lj.si, http://www.fkkt.uni-lj.si)
bUniversity of Regensburg, Institute of Physical and Theoretical Chemistry, Universitätsstr. 31, D-93040, Regensburg, Germany
cUniversity of Graz, Institute of Chemistry, Heinrichstr. 28, A-8010 Graz, Austria
Structural properties of ternary systems composed of nonionic surfactant dodecyl-poly(ethylene oxide-23) ether (C12E23, commercial name: Brij 35), water and various alcohols from ethanol to 1-decanol have been investigated using small-angle X-ray scattering (SAXS) and dynamic light scattering (DLS) techniques. All measurements were performed at the temperature 25°C. SAXS experimental data were put on absolute scale using water as a secondary standard. The data of water-rich mixtures at low to moderate surfactant concentrations were evaluated using the generalized indirect Fourier transformation method (GIFT), which is based on the simultaneous determination of the intra- and interparticle scattering contributions. In this way, the size and the shape of interacting scattering particles in real space could be deduced. The systems with a relatively low surfactant concentration (5 mass %) were studied the most extensively. In these cases, the water-rich regions of the phase diagrams could be investigated into more detail, since in the alcohol-rich regions problems with the GIFT evaluation of the SAXS data were encountered. The results reveal the inner structuration of the complex ternary systems of our present interest. In parallel, they also indicate that the longer chain alcohols actually behave as real oil phases in the studied systems, as one might expect, and also confirm the well-known properties of different short to medium chain alcohols that act as co-solvents and/or co-surfactants in microemulsion systems depending on their chain length.
P. Matějíčeka, M. UCHMANa, M. ŠTĚPÁNEKa, M. ŠPÍRKOVÁb, K. PROCHÁZKAa
aDepartment of Physical and Macromolecular Chemistry, Faculty of Science, Charles University, Albertov 2030, 128 43 Praha 2, Czech Republic (matej/vivien.natur.cuni.cz)
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, 162 06 Praha 6, Czech Republic
Block copolymer polystyrene-block-poly(methacrylic acid), PS-PMA, self-assembles into fairly monodisperse micelles when dissolved in 1,4-dioxane-rich mixture with water. Poly(2-vinylpyridine), PVP, chains interact strongly with micelle-shell blocks via hydrogen bond in organic media, and form large polydisperse clusters in the solution. When water content in the solution is increased by dialysis into a basic aqueous buffer, the clusters split into fairly monodisperse particles.
The micelles prepared as described above, were studied by a combination of light scattering, atomic force microscopy and fluorescence methods for various solution compositions, pH of aqueous buffers, PVP content and PVP chain length with respect to that of PMA blocks.
M. JAMRÓZ-PIEGZAa, Ph. DIMITROVb, B. TRZEBICKAa, A. DWORAKa
a Polish Academy of Science, Institute of Coal Chemistry, ul. Sowińskiego 5, 44-100 Gliwice (marysia/karboch.gliwice.pl)
bBulgarian Academy of Science, Institute of Polymers, Sofia, Bulgaria
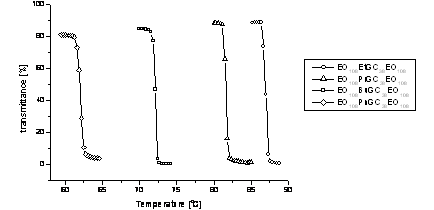 |
New temperature sensitive ABA amphiphilic block copolymers
consisting of hydrophilic poly(ethylene oxide) (A) and
hydrophobic: poly(ethyl glycidyl carbamate) (EO108EtGC38EO108),
poly(propyl glycidyl carbamate) (EO108PrGC38EO108),
poly(butyl glycidyl carbamate) (EO108BuGC38EO108)
or poly(pentyl glycidyl carbamate) (EO108PnGC38EO108)
(B) blocks were synthesized. Anionic polymerization and chemical
modification reactions were used. The clouding processes of
obtained block copolymers was studied by UV-VIS spectroscopy. The
cloud point varied from 65 to 87 oC, and decreased
with increasing chain length of substituted isocyanate (FIG.1).
FIG. 1 Transmittance at 500 nm vs temperature curves for 10 g/L aqueous solutions of the block copolymers.
The self association behavior of investigated block copolymers was studied using UV-VIS spectroscopy and dynamic and static light scattering. Obtained copolymers spontaneously form micelles in aqueous media. The type of isocyanate used has an significant influence upon the critical micellization concentration (CMC). Also the temperature influence upon the CMC were investigated. The radius of gyration (Rg), hydrodynamic radius (Rh), molar mass of the micells Mwmicelles and the agreggation number Nagg was also determined at different temperatures. The sizes of structures formed increases with increasing alkyl chain length of isocyanate used.
M. SCHUMACHERa, M. RUPPELa, O. COLOMBANIa, F. PLAMPERa, M. DRECHSLERa, A.H. MÜLLERa , S. LINDHOUDb
aMakromolekulare Chemie II, Universiät Bayreuth, D-95440 Bayreuth, Germany
bPhysical Chemistry and Colloid Science, Wageningen University, The Netherlands
We present the investigation of complex formation between highly functionalized water-soluble silsesquioxane-nanoparticles with star-shaped polyacrylic acid (PAA) and micelles of poly(n-butyl acrylate)-block-poly(acrylic acid) (PnBA-b-PAA). The synthesis of the nanoparticles with a diameter of 3 nm is reported as well as their labelling with a fluorescent dye utilized for Fluorescence Correlation Spectroscopy (FCS) studies.
Complex formation and its pH-dependence are studied by Dynamic Light Scattering (DLS) titrations, potentiometry, conductometry and 2D-NMR techniques. FCS and cryogenic Transmissions Electron Microscopy (cryo-TEM) sustain the formation of a new type of organic-inorganic nano-hybrids.
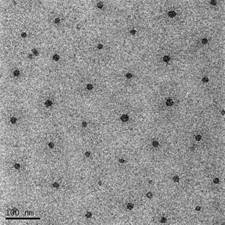
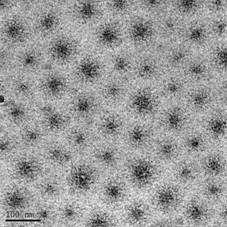
Figure 1. Cryo-TEM of PnBA90-b-PAA300 (5 g/l, left image; only the core of the micelle is visible) and after complex formation with the functionalized silsesquioxane nanoparticles (5 g/l, right image) in water at pH 7 after staining with CsOH (scale bar: 100 nm).
K. STUBENRAUCHa, C. MOITZIb, A. LEXa, G. FRITZb, G. TRIMMELa
aInstitute for Chemistry and Technology of Organic Materials, Graz University of Technology, Stremayrgasse 16, 8010 Graz, Austria, k.stubenrauch/tugraz.at
bInstitute of Chemistry, University of Graz, Heinrichstrasse 28, 8010 Graz, Austria
Amphiphilic block copolymers with two covalently bonded incompatible blocks form defined micelles in selective solvents. Ring opening metathesis polymerization (ROMP) is a powerful method of synthesizing block copolymers. The development of well defined initiator systems during the last 20 years opened the way for the preparation of well-defined block copolymers with a narrow distribution of molecular weights.
We present the preparation of precursor block copolymers by ROMP consisting of norbornene dicarboxylic acid di-tert-butylester, as disguised hydrophilic block, and norbornene dicarboxylic di-methylester as hydrophobic block. The polymers were subsequently transformed into amphiphilic block copolymers by selectively cleaving the tert-butyl esters with trifluoroacetic acid.
The polymers were fluorescence labelled by covalently attaching pyrene. The fluorescence of pyrene strongly depends on its environment. Upon absorption of a photon, an excited pyrene can either emit fluorescence as a single dye in the blue or can form an excimer which emits in the green. So the micelle formation can be detected by the excimer formation of pyrene.
The self-assembly in solution of these materials was also studied by dynamic light scattering (DLS). Ethanol is a selective solvent for the acid block and was therefore used for investigation of the micelle formation. It was shown that the dimensions of the micelles can be tailored by the composition of the block copolymer and the micelles formation itself can be detected sensitively by the excimer formation of pyrene.
A. WALTHER*, M. DRECHSLER, A. H. E. MÜLLER
Makromolekulare Chemie II and Bayreuther Zentrum für Kolloide und Grenzflächen, University of Bayreuth, D-95440 Bayreuth, Germany.
Janus structures are colloids, polymeric assemblies or low molecular weight compounds (dendrimers or metal complexes), which possess two segregated compartments of different chemistry or polarity (see figure 1).
Due to the non-centrosymmetric arrangement of the corona, the particles can undergo self-assembly processes into superstructures even in good organic solvents. This contribution reports on cryogenic transmission electron microscopy studies of the unimolecular structures and superstructures formed in organic solution (THF) and water of spherical, cylindrical and sheet-like Janus particles. In this context, cryo-TEM is one of the only in-situ methods providing sufficient information about colloidal aggregates of moderate or high polydispersity. With this first report of cryo-TEM studies in THF, it was possible to deduce further information about the aggregation patterns of Janus micelles, Janus cylinders and Janus sheets, which cannot be obtained in a similarly straightforward approach by means of other in-situ techniques like scattering experiments.
A. MAVROUDISa, A. AVGEROPOULOSb, N. HADJICHRISTIDISa, M. PITSIKALISa, E.L. THOMASc , D.J. LOHSEd
aDepartment of Chemistry, University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece (pitsikalis/chem.uoa.gr, http://www.chem.uoa.gr/courses/polymers/PG_Polym_Index.htm )
bDepartment of Materials Science and Engineering, University of Ioannina, Panepistimiopolis, 45110 Ioannina, Greece
cDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
dCorporateStrategic Research Labs, ExxonMobil Research & Engineering Co., Annandale, NJ 08801, USA
In the present communication 6-miktoarm star copolymers [PS(P2MP)5] of styrene (S) and 2-methyl-1,3-pentadiene (P2MP) were synthesized by anionic polymerization and controlled chlorosilane chemistry, using high vacuum techniques. Molecular characterization, by several methods revealed high molecular weight/composition homogeneity, whereas structural analysis by DSC, TEM and SAXS showed highly ordered structures. Pronounced deviations from predictions based on Milner's morphological model were observed and were attributed to the crowding of the arms for these highly branched molecules.
* The project was co-financed within Op. Education by the ESF (European Social Fund) and National Resources(program Pythagoras)
A. KARATZAS, M. PITSIKALIS, N. HADJICHRISTIDIS
Department of Chemistry, University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece
(pitsikalis/chem.uoa.gr, http://www.chem.uoa.gr/courses/polymers/PG_Polym_Index.htm )
PS-b-PI block copolymers of polystyrene (PS) and polyisoprene (PI) were prepared having either at the PS or the PI chain end functional groups that may form multiple hydrogen bonds. Two series of ω-functionalized diblock copolymers carrying hydroxyl end groups PS-b-PI-OH and PI-b-PS-OH were synthesized by living anionic polymerization with similar molecular weights and different compositions. Through the hydroxyl group the functional 2-ureido-4-pyrimidone (UPy) end-group was incorporated in each copolymer. The UPy group dimerizes strongly in a self - complementary array of four cooperative hydrogen bonds. The bulk properties of the copolymers properties were studied by DSC and TGA. The micellization behavior was studied in decane, which is selective for the PI blocks. The formation of supramolecular structures through the interactions of the micelles was evidenced when the hydrogen bond forming group was located at the PI chain end, which forms the micellar corona. Intermicellar interactions were not obtained when the UPy groups were placed at the PS chain ends, which is the micellar core forming block.
M. MILNERA, I. ZUSKOVÁ, K. PROCHÁZKA
Department of Physical and Macromolecular Chemistry, Charles University Prague, Hlavova 8, 12843 Prague 2, Czech Republic, milnera/gmx.net
Kinetically frozen polystyrene-block-poly(methacrylic acid), PS-PMA, micelles were prepared by stepwise dialyses into aqueous 1,4-dioxane solutions with increasing water content. Final dialysis was done into a TAPS-buffer of pH 9.12 and ionic strength 0.02 mol/L. The electrophoretic mobility of PS-PMA micelles which report on the unscreened charge (and on z-potential) was investigated by capillary zone electrophoresis (CZE). Thiourea was added as marker for the electro osmotic flow (EOF). The electrophoretic curves show broad micellar peaks (see Fig. 1a). The mobility of the micelles was measured as a function of pH (Fig. 1b). Due to the opposite velocities of the micelles and the EOF a double side injection method had to be developed. Quasi elastic light scattering (QELS) was used to determine the hydrodynamic radius of the micelles as a function of pH. Combining the results of CZE and QELS, the net charge of the micelles was estimated. The z-potentials vary from 35.7 mV at pH 5.24 to 43.1 mV at pH 9.12. The performed experiments show that CZE is a suitable technique for study of polyelectrolyte micelles.
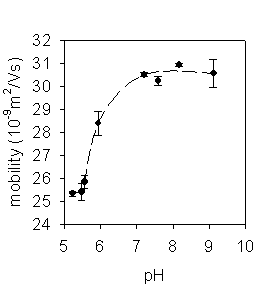
Fig. 1
Č. KOŇÁK, M. HRUBÝ
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic (konak/imc.cas.cz)
Solutions of poly(N-isopropylacrylamide) (PNIPA) with added ionic and non-ionic surfactants (SR) were investigated by light scattering methods at temperatures 15 - 40 oC. The formation of well defined nanoparticles of PNIPA was observed on heating at low surfactant additions.
The following surfactants were used: Sodium dodecyl sulfate (SDS), 1-Hexadecylpyridinium chloride monohydrate (HDPC) and Pluronic F-68 (PL), Hexadecyltrimethylammonium bromide (CTAB), Brij 97 (B97) and 98 (B98).
Poly(N-isopropylacrylamide) (PNIPA) polymers with viscosity-average molecular weight Mη = 67 000, 122 000 and 220 000 were used for investigation.
The weight-average molecular weight, Mw, and hydrodynamic radius, Rh, of nano-particles obtained at 40 oC are shown for several SR/PNIPA solutions in Table 1.
Table 1. Characteristics of nanoparticles
| Surfactant | Mw´10-6 | Rg [nm] | Rh [nm] | DRh/Rh |
| SDS | 13 | 18 | 25 | 0.17 |
| CTAB | 14 | 20 | 27 | 0.15 |
| HDPC | 14 | 25 | 33 | 0.23 |
| PL | 420 | 63 | 84 | 0.07 |
| B98 | 570 | 87 | 112 | 0.10 |
| B97 | 880 | 116 | 136 | 0.09 |
Mh = 122 000, cPNIPA = 5 x 10-4 and cSR = 5 x 10-5 g mL-1
The nanoparticle parameters can be varied by PNIPA and surfactant concentra-tions and the molecular weight of PNIPA in a wide range of Mw and Rh values. An interpretation based on stabilization of PNIPA nuclei by surfactants was suggested.
Financial support of the Grant Agency of the Academy of Sciences of the Czech Republic (grants A100500501, A4050403 and A400480616) is acknowledged.
J. KŘÍŽ, J. DYBAL, J. BRUS
Department of Structure Analysis, Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic (kriz/imc.cas.cz, https://www.imc.cas.cz/en/imc/struanal )
Poly-4-vinylpyridine (PVP) effectively substitutes monomeric pyridine bound by hydrogen bonds to poly-4-vinylphenol (PVF)forming thus a macromolecular complex precipitating from a THF solution. The prevailing structure of the PVP-PVF complex is regular. As predicted by ab initio SCF calculations and confirmed by solid-state NMR, it forms sheets of interleaved PVF and PVP strands.
The cooperativity of H-bonds in PVP-PVF interaction is confirmed by the fact that PVP units substitute pyridine molecules even in their 20 mol/mol (or higher) excess. It is of a higher order in the sense that higher order terms in the expression
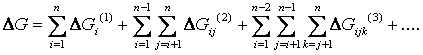
contribute to the negative DG. This can be illustrated by the sigmoidal dependence of the conversion a of VP units on the polymerization degree of PVP. Somewhat surprisingly, a less pronounced but still obvious sigmoidal dependence of a on the b=VP/VF molar ratio exists in the range of bbetween 0 and 1. Considering the structure of the complex, we propose the cooperativity to be "two-dimensional", the one dimension being along the PVP chain and depending on its Pn, the second being along the width of the sheet of the complex and depending on b.
According to our results, the driving force of the present cooperativity is (i) the entropy gain of the liberated small ligand (pyridine) molecules (contribution to DGi(1)) and (ii) the proximity effect of neighboring groups (contribution to DGij(2) and higher terms) influencing the statistics of encounter of the reacting VP and VF groups.
Acknowledgement: This work was sponsored by the Grant Agency of the Academy of Sciences of the Czech Republic under the Grant IAA 400500604
S. KUKLA, V. CIMROVÁ, P. PAVLAČKOVÁ, D. VÝPRACHTICKÝ
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic (kukla/imc.cas.cz, cimrova/imc.cas.cz, pavlackova/imc.cas.cz, vyprach/imc.cas.cz )
Polymer ligands containing a covalently bonded quinolinone fluorophore and COOH (L1) or COONa (L2) binding sites were synthesized by the reaction of poly[styrene-alt-(maleic anhydride)] with 7-amino-4-methylquinolin-2(1H)-one and methanol and subsequent neutralization. The ligand-to-metal energy transfer (Fig. 1) and ligand binding properties in a series of [Tb(III)-ligand] complexes were investigated by steady-state and time-resolved luminescence spectroscopy in methanol or deuterated methanol. The intensity of the long-lived terbium(III) ion emission at 490, 545, 585 and 620 nm was greatly enhanced upon addition of L1 or L2. Based on the differences in luminescence data obtained for [Tb(III)-L1] and [Tb(III)-L2] complexes, a qualitative model for the interaction of terbium(III) ion with L1 and L2 is put forward. The experimental luminescence decay curves were double-exponential (t1, t2) with predominating longer component (rel B1 > 85 %) for both [Tb(III)-L1] and [Tb(III)-L2] complexes.
We thank the Grant Agency of the Czech Republic for support (grant 203/04/1372) and the Grant Agency of the Academy of Sciences of the Czech Republic (grant IAA4050409).
I. MYSKOVAa, O. BUDISHEVSKAa, S. VORONOVa
aInstitute of chemistry and chemical technologies, National university "Lvivska polytechnika", str. Bandery 12, Lviv, 79013, Ukraine (budish/polynet.lviv.ua)
Surface modification of polymeric disperse systems allows to create new polymeric materials with valuable properties. Radical grafting of heterofunctional polymers to polymeric surface is promising method of modification.
Copolymer octene-co-maleic anhydrid-co-tert-butylperoxymethylmaleate (O-MA-TBPMM) was used as emulsifier-initiator for polymeric dispersions with activated surface of dispersoid.
O-MA-TBPMM is a polymer with alternate structure. In its molecule oktene links alternate with maleic anhydrid (MA) links or tert-butylperoxymethylmaleate links. O-MA-TBPMM is not water-soluble. But after hydrolisis of MA links with sodium hydroxide presence its well dissolved in water. Water-soluble sodium salts of octene-co-maleic acid-co-tert-butylperoxymethylmaleate (O-MAS-TBPMMS) can lover surface tension and are effective in styrene and others monomers solubilizations.
O-MAS-TBPMMS was used as emulsifier-initiator in styrene emulsion polymerization. Were obtained latexes with particle on surface of which were grafted O-MAS-TBPMMS chains.
Emulsion polymerization of styrene was initiated by radicals which are formed by primary-tertiary peroxide groups thermo-decomposition. Polymerization of styrene is providing with chain-transfer reactions to polystyrene macromolecules and recombination of O-MAS-TBPMMS polymer radicals and polystyrene-radicals.
Graft chains of O-MAS-TBPMMS to particles surface contain active primary-tertiary peroxide groups. This groups was used for grafting of polyacrylonitrile to particle surface.
Acrylonitrile was added to polystyrene latex and polymerization was carrying out with initiated by method "from surface". Polymer dispersions with "core-shell" structure were obtained. Graft-polyacrylonitrile quantity in pure latexe polymer was calculated by means of nitrogen analysis.
E.N. KONYUSHENKOa, M. TRCHOVÁa, I.SAPURINAb, J. STEJSKALa
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky sq. 2, CZ-162 06 Prague 6, Czech Republic (elenak/imc.cas.cz);
bInstitute of Macromolecular Compounds, Russian Academy of Sciences, St. Petersburg 199004, Russia
Aniline has been oxidized with ammonium peroxydisulfate1 in the aqueous solution of acetic acid. The oxidation had two subsequent phases: the oxidation of neutral aniline molecule at low acidity was followed by the oxidation of anilinium cation after the acidity became higher. Both phases of oxidation gave different products, aniline oligomers and polyaniline nanotubes, respectively. Sulfuric acid is a by-product of oxidation and pH decreases as the reaction proceeds. A high-molecular-weight polyaniline is produced as nanotubes in acidic media2 (Fig. 1).
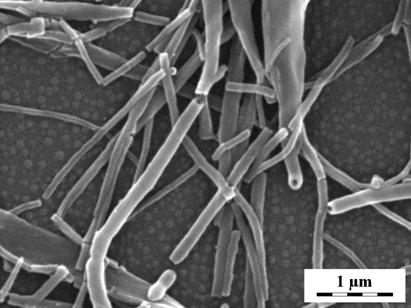 The nano-sized crystallites on the surface of
aniline oligomers serve as starting templates for the nucleation
of PANI nanotubes. It is proposed that the further growth of
nanotubes proceeds by the self-organization of polymer chains.
PANI nanotubes have a typical outer diameter of 100-200 nm, inner
diameter of ca 50 nm, and length extending to a few
micrometers. Transfer of electrons between the aniline and
oxidant molecules under-going a redox reaction through the
conducting PANI structure is proposed to be of fundamental
importance in the formation of extended morphologies, like
nano-tubes.
The nano-sized crystallites on the surface of
aniline oligomers serve as starting templates for the nucleation
of PANI nanotubes. It is proposed that the further growth of
nanotubes proceeds by the self-organization of polymer chains.
PANI nanotubes have a typical outer diameter of 100-200 nm, inner
diameter of ca 50 nm, and length extending to a few
micrometers. Transfer of electrons between the aniline and
oxidant molecules under-going a redox reaction through the
conducting PANI structure is proposed to be of fundamental
importance in the formation of extended morphologies, like
nano-tubes.
1. Stejskal, J.; Gilbert, R.G. Pure Appl. Chem. 2002, 74, 857.
2. Konyushenko, E.N.; Stejskal, J.; Šeděnková, I.; Trchová, M.; Sapurina, I.; Cieslar, M.; Prokeš, J. Polym. Int. 2006, 55, 31.
G. ĆIRIĆ-MARJANOVIČa, I. ŠEDĚNKOVÁb, S.V. SOLOSINb, J. STEJSKALb, M. TRCHOVÁb
aFaculty of Physical Chemistry, University of Belgrade, 11001 Belgrade, Serbia and Montenegro
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky sq. 2, CZ-162 06 Prague 6, Czech Republic (trchova/imc.cas.cz)
 The course of aniline oxidation with ammonium
peroxydisulfate in water in the absence of acid has been
investigated. The reaction was terminated at various times and
the intermediates collected. In addition to polyaniline
precipitates, also the films deposited in situ on silicon
windows have been studied. The kinetics of polymerization is
controlled by the acidity level, which changes during the
polymerization from pH 8 to a final value close to pH 1 as
sulfuric acid is generated as a by-product. The oxidation has two
distinct exothermic phases. Gel-permeation chromatography
suggests that aniline oligomers are produced at first at high pH,
polyaniline formation follows after the pH becomes sufficiently
low. The growth of polyaniline nanotubes was observed by optical
microscopy and confirmed by electron microscopy (Fig. 1). The
molecular structure of the precipitated reaction intermediates
was studied in detail by FTIR spectroscopy. Oxidation products
are partly sulfonated and they contain phenazinium units. Aniline
oligomers are more soluble in chloroform than polymer fraction
containing nanotubes.1
The course of aniline oxidation with ammonium
peroxydisulfate in water in the absence of acid has been
investigated. The reaction was terminated at various times and
the intermediates collected. In addition to polyaniline
precipitates, also the films deposited in situ on silicon
windows have been studied. The kinetics of polymerization is
controlled by the acidity level, which changes during the
polymerization from pH 8 to a final value close to pH 1 as
sulfuric acid is generated as a by-product. The oxidation has two
distinct exothermic phases. Gel-permeation chromatography
suggests that aniline oligomers are produced at first at high pH,
polyaniline formation follows after the pH becomes sufficiently
low. The growth of polyaniline nanotubes was observed by optical
microscopy and confirmed by electron microscopy (Fig. 1). The
molecular structure of the precipitated reaction intermediates
was studied in detail by FTIR spectroscopy. Oxidation products
are partly sulfonated and they contain phenazinium units. Aniline
oligomers are more soluble in chloroform than polymer fraction
containing nanotubes.1
1. M. Trchová, I. Šeděnková, E. N. Konyushenko, J. Stejskal, P. Holler, G. Ćirić-Marjanović, J. Phys. Chem. B. 2006, in press.
Acknowledgment. The authors thank the Grant Agency of the Academy of Sciences of the Czech Republic (A4050313 and A400500504) and the Ministry of Science and Environmental Protection of the Republic of Serbia (142047) for financial support.
FIG. 1. The nanotubular morphology of oxidation products
J. HORSKÝ, J. PODEŠVA
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, 162 06 Praha 6, Czech Republic
(horsky/imc.cas.cz)
Poly(methacrylic acid) (PMAA) is well known for its transition between "closed" and "open" conformations occurring in aqueous solution upon neutralization. As a consequence, the Henderson-Hasselbalch (HH) plot of pH vs log[(1-a)/a], is formed by two distinct linear parts for PMAA while no break is usually observed with other weak polyelectrolytes such as poly(acrylic acid) (PAA).2 Although it is clear from a PMAA/PAA comparison that the presence of hydrophobic methyl groups is responsible for the transition, the phenomenon is not fully understood even after more than forty years of research.
Recently, the PMAA conformational transition was studied indirectly, following the behavior of the 1,1¢-binaphthalene (BN) label,3 which was found to be accommodated in the cavity of g-cyclodextrin (gCD) only at pH > 6, i.e., above the pH region of the conformational transition. Cyclodextrins (CDs) form inclusion complexes with various linear polymers. Therefore, we examined possibility that gCD interacts not only with BN but also with PMAA itself.
Addition of 3 % of gCD to PMAA solution changed the HH plot to
that of usual weak polyacid, i.e., without any break, thus
clearly demonstrating that gCD interacts with PMAA in a specific
manner. The transition was not altered by addition of 3 % of
glucose, which corresponds to the cyclodextrin constitution
units. The coincidence of HH plots in the absence and presence of
gCD was found at low pH where PMAA is in "closed"
conformation. Although the observation is in agreement with the
data obtained with BN-labeled PMAA,3 it is somewhat
surprising because the "closed" conformation is
considered as responsible for anomalous PMAA behavior in aqueous
solution.2 Here, however, the HH plot is
"normalized" by making proton dissociation from the
"open" conformation more difficult. The mechanism,
including the mode of gCD/PMAA interaction
(main- chain vs. side-chain inclusion), has yet to be elucidated.
1Support of the Academy of Sciences of the Czech Republic (GAAV 040503) is acknowledged.
2 J. C. Leyte, M. Mandel: J. Polym. Sci., A2, 1879 (1964).
3 S. Y. Yang, G. Schultz, M. M. Green, H. Morawetz: Macromolecules 32, 2577 (1999).
J. PLEŠTILa, I. KRAKOVSKÝb, H. POSPÍŠILa, Č. KOŇÁKa, Z. SEDLÁKOVÁa, M. ŠLOUFa, L. ALMÁSY,c A.I. KUKLINd
aInstitute of Macromolecular Chemistry, Academy of Science of the Czech Republic, Heyrovský Sq. 2 , 162 06 Prague , Czech Republic
bDepartment of Macromolecular Physics, Faculty of Mathematics and Physics, Charles University, V Holešovickách 2,
180 00 Prague 8, Czech Republic
cKFKI Research Institute for Solid State Physics and Optics, P.O.Box 49, Budapest, H-1525 Hungary
dFrank Laboratory of Neutron Physics, Joint Institute for Nuclear Research, 141 980 Dubna, Russia
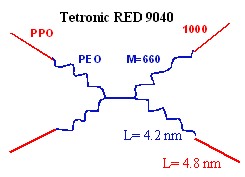 Structure of mixtures of Tetronic RED 9040, a
reverse-type (with hydrophobic terminal blocks) octablock star
copolymer with four poly(ethylene oxide)/poly(propylene oxide)
(PEO/PPO) arms attached to the ethylenediamine core, with normal-
and reverse-type PEO/PPO triblock copolymers was investigated in
D2O as a function of the mixture composition and
temperature.
Structure of mixtures of Tetronic RED 9040, a
reverse-type (with hydrophobic terminal blocks) octablock star
copolymer with four poly(ethylene oxide)/poly(propylene oxide)
(PEO/PPO) arms attached to the ethylenediamine core, with normal-
and reverse-type PEO/PPO triblock copolymers was investigated in
D2O as a function of the mixture composition and
temperature.
Combination of SANS, DLS and LS techniques lead to the following findings: At low temperatures and concentrations the copolymers are molecularly dispersed. Upon increasing temperature water becomes a bad solvent for PPO and the copolymer self-assembles to form micron-sized particles with a lamellar internal nanostructure (multilamellar vesicles). In this respect the associative behaviour of Tetronic differs from that of the vast majority of amphiphilic copolymers in selective solvents which form nano-sized core/corona micelles.
The thermosensitive vesicles were stabilized by forming a PMMA shell on their surface. This made it possible to study the vesicles using TEM. In agreement with the LS results, micron-sized spherical particles were observed.
The addition of a triblock PEO/PPO copolymer leads to the transition from multi-lamellar to unilamellar structure. The structure development after temperature and composition jumps was investigated in time-resolved SANS experiments.
M. SEDLÁK
Institute of Experimental Physics, Slovak Academy of Sciences,
Watsonova 47, 043 53 Košice, Slovakia
E-mail: marsed/saske.sk
Recent developments in the investigation of the domain structure of polyelectrolyte solutions due to physically non-trivial attraction between macroions with like charges will be presented. They can be divided into several areas.
Stability of domains in solutions prepared in a classical way (direct dissolution of bulk polymer in water) were studied over extremely long time intervals ranging up to three years. Slow kinetic changes were observed with intensities of these time effects varied from strong to none (completely stable structures without any changes). Results show that multimacroion domains survive in solution over very long time intervals. The infinite (or at least extremely long) life-times of loose domains also suggest thermodynamic attraction between polyions.
Mechanical properties and stability of multi-macroion domains were studied in detail upon passage through obstacles created by nanosized pores comparable to domain sizes. Size distribution of multimacroion domains in a natural state (without mechanical treatment) was obtained for the first time. It was shown that domains cannot be characterized as dynamic large-scale concentration fluctuations, but rather as long-lived associates of macroions, which can be mechanically affected (disintegrated into smaller ones but also coalesced into larger ones).
It was shown for the first time that there is solvent inside domains. Until now, no scattering experiment on polyelectrolyte domains was able to discriminate between tightly packed structures and loose structures with solvent inside.
N.M. Zhunusbekova, N.B. Sarova, T.K. Jumadilov, E.A. Bekturov
Laboratory of physics-chemical methods researches, Institute of Chemical sciences named by A.B. Bekturov, Ministry Education and Science Republic of Kazakhstan, Walikhanov str. 106, Almaty, 050010, Republic of Kazakhstan (znazym/mail.ru)
We have been extracted porphyrin (a mix of a+b phaeophytines) from plant raw materials of Kazakhstan. Gel of polyacrilic acids obtained by means of radical polymerization of polyacrilic acids. Ternary complexes of hydrogel with metal and phaeophytin like "sandwich" form obtained in the isopropyl alcohol solution was studied. It is shown that a necessary condition of complexformation is presence of a coordinating metal ion.
Kinetic of ternary complexformation at different concentration of phaeophytin (С=10-5-10-4 mol/l) and at the various contents of metal (1:55, 1:75, 1:135) in isopropyl alcohol has been investigated. It is shown that formation of a complex with gel at different ratio metal:phaeophytin n=1:55, 1:75, 1:135 and at different concentration of phaeophytin accompanied by the deep counteraction of hydrogel.
Process of counteraction of gel with increase in concentration of metal and phaeophytin can be observed visually. There is a general tendency of decrease of swelling degree in a number of researched metals, thus the degree of swelling accepts values 7,7>7,45>7,27 for ions Co>Ni>Fe (1:55); 7,08>7,04>6,94 for ions Fe>Co>Ni (1:75); 6,85>6,65>6,3 for ions Co>Ni>Fe (1:135).
Comparison of the received results shows that value of degree of linkage θ lin of Fe-phaeophytin with hydrogel is more than at nickelphaeophytin and cobaltphaeophytin. In this case the maximal θ lin accepts values 0,97>0,44>0,36 for ions Fe>Ni>Co (n=1:75), and at n=1:135 the maximal degree of linkage 0,58>0,48>0,42 for ions Ni>Fe>Co. It can be connected to difficulty of introduction in a phaeophytin ligand plane of Со2 + ion.
Sorrel acid oxidation catalyzed by ternary complexes based on hydrogel - Fe - phaeophytin was carried out. A high stability of the catalytic site and high speed of sorrel acid oxidation reaction was observed with the ternary complexes.
L. STAROVOYTOVA
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
It is well known that some acrylamide-based polymers, including poly(N-isopropylacrylamide) (PIPAAm) and poly(N-isopropylmethacrylamide) (PIPMAm), and some other polymers like poly(vinyl methyl ether) (PVME), in aqueous solutions exhibit a lower critical solution temperature (LCST), i. e., they are soluble at low temperatures but heating above LCST results in phase separation (precipitation of polymer). Such polymers have hydrophilic part, which can stabilize the aqueous solution by forming hydrogen bonds with water molecules, and hydrophobic parts, which destabilize the solution by altering water structure and decreasing the entropy of water. On the molecular level, the coil-globule transition is induced by a small change in the balance between the hydrogen bonding and the hydrophobic interactions. Therefore, it is important that the phenomenon is closely related to the hydration of the polymer and the structure of water around the polymer chain. Structural changes and physical interactions are more easily studied in polymer solutions than gels, especially by using modern spectroscopic methods. In the present work we concentrated on the dehydration process during the temperature-induced phase separation in mixtures of several thermoresponsive polymers. Moreover, a connection between the state of the globular-like structures (hydrated or dehydrated) of the component with lower LCST (PVME, PIPAAm) and the phase separation of the component with higher LCST (PIPMAm) in D2O solutions of PIPMAm/PVME and PIPMAm/PIPAAm mixtures was studied. From the methodical point of view, we combined the measurements of time dependences of spin-spin relaxation times T2 of residual HDO molecules and temperature dependences of absolute integrated intensities of polymer signals in high-resolution 1H NMR spectra, that make it possible to determine the temperature dependences of the phase-separated fraction.
This work was supported by the Academy of Sciences of the Czech Republic (project AVOZ 40500505).
M. ŠTĚPÁNEKa, R. ŠACHLa, M. UCHMANa, M. ŠPÍRKOVÁb, K. PROCHÁZKAa
aDepartment of Physical and Macromolecular Chemistry, School of Science, Charles University, Albertov 2030, CZ-128 43 Praha 2, Czech Republic (e-mail: stepanek/natur.cuni.cz)
bInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
Aqueous solutions of self-assembled nanoparticles formed by biocompatible block copolymers of poly(ethylene oxide)-block-poly(e-caprolactone) differing in the length of the hydrophobic block have been prepared from solutions of the copolymers in a mild selective solvent, tetrahydrofuran (THF)/water mixture, by (i) mixing of the copolymer solution in THF/water with an excess amount of water and (ii) the removal of THF by dialysis of the solution against water. The nanoparticles have been characterized by static and dynamic light scattering and atomic force microscopy imaging and the kinetics of pyrene release from the nanoparticles to water has been studied using pyrene fluorescence measurements.
The results show that the nanoparticles are in a kinetically-frozen, non-equilibrium state in aqueous solutions and their size, molar mass and other properties thus depend not only on length of the blocks, but also on the preparation protocol, especially on the composition of the initial mild selective solvent.
I. K. VOETS, A. DE KEIZER, M.A. COHEN STUART
Laboratory of Physical Chemistry and Colloid Science, Wageningen University, Wageningen, Netherlands
Upon mixing of aqueous solutions of two oppositely charged double hydrophilic block copolymers (PAA42-PAAm417 and P2MVP42-PEO446), mixed core-shell micelles are formed spontaneously (1mM NaNO3, pH=7.73, f+ = [P2MVP]/[PAA]+[P2MVP])=0.5). The core is a complex coacervate of PAA and P2MVP, while the corona consists of a PEO and PAAm brush. The tendency of PAAm and PEO to phase segregate inside the corona, induces a disc shaped morphology where PEO and PAAm reside on opposite faces of the micelle, as evidenced by DLS, SLS, Cryo-TEM, SANS and 1H NMR NOESY. This study is the first account of the spontaneous formation of reversible Janus (after the double-faced Roman deity Janus) micelles in aqueous solutions through a combination of associative and segregative micro phase separation.
Fig.1. Schematic representation of a reversible, disc shaped Janus micelle.
The complex coacervate core, consisting of PAA and P2MVP groups, is marked orange, the two microphase separated coronal hemispheres green (PEO) and blue (PAAm).
B. HOFS, I.K. VOETS, A. DE KEIZER, M.A. COHEN STUART
Laboratory of Physical Chemistry and Colloid Science, WUR, P.O. Box 8038, 6700 EK, Wageningen, The Netherlands (bas.hofs/wur.nl, http://www.pcc.wur.nl/ )
Complex coacervate core micelles (C3Ms) can form from a water-soluble diblock copolymer with a charged block (polymer 1) and an oppositely charged polyelectrolyte (polymer 2)(1). The core of the micelles is composed of the oppositely charged polyelectrolytes, whereas the neutral blocks form a stabilizing corona. By partially replacing the polyelectrolyte with a second water-soluble diblock copolymer species (polymer 2) the number of neutral blocks per micelle increases, decreasing the size of the C3Ms. A simple model for the radius of such C3Ms is proposed, based on the area A of the interface between the core and the corona, the volume of the core, Vcore, and the volume of the corona Vcorona. The model, even though it is simplistic, describes the change in the size of C3Ms with increasing diblock copolymer percentage rather well (fig. 1).
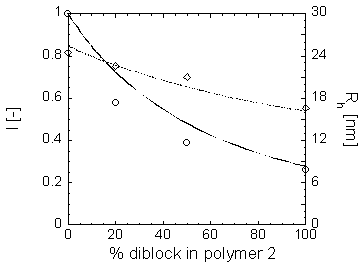
Fig. 1 Comparison of the relative light scattering intensity I and hydrodynamic radii Rh, obtained with the model and experiments. I (solid line, ○) and Rh (dotted line, à) are given as a function of the percentage of diblock copolymer (PDMAEMA45-b-PGMA90, polyelectrolyte is PDMAEMA150) in polymer 2. Lines are from the model, symbols are from experiments.
1. Cohen Stuart, M. A., Besseling, N. A. M. & Fokkink, R. G. (1998) Langmuir 14, 6846-6849.
P. ČERNOCH1, E. KRUMBHOLCOVÁ1, L. MARTINOVÁ2, M. PŘÁDNÝ1, P. ŠTĚPÁNEK1, J. MICHÁLEK1
1 Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovský Sq. 2, 162 06 Prague 6, Czech Republic
2 Technical University, Faculty of Textile Engineering, Hálkova 6, 461 17 Liberec, Czech Republic
2-Hydroxyethyl methacrylate (HEMA) polymers have been the subject of intensive research because of their good biocompatibility and other properties suitable for use in biomedical applications. We have synthesized and characterized copolymers of HEMA with 2-ethoxyethyl methacrylate (EOEMA), which can be used in manufacture of nanofibre materials by electrospinning. The copolymers and their nanofibres are quite stable in water environment and hence can find various application such as in tissue engineering and nanofiltration. The electrospinning itself, however, is a sensitive method proceeding under finely tuned conditions; so detailed examination of the solutions was performed. We found that at higher concentration of the copolymers in ethanolic solution clustering of copolymer molecules occurs, which has a striking influence on the course and timing of the electrospinning process.
We acknowledge financial support by the Ministry of Industry and Trade of the Czech Republic (project 1H-PK 2/46).
I. KROUTILOVÁ, L. MATĚJKA, J. PLEŠTIL, J. BRUS, M. ŠLOUF
Department of Polymer Networks and Mechanical Properties, Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky sq.2, 162 06 Prague 6, Czech Republic, kroutili/imc.cas.cz
Along with increased use of synthetic polymers more demanding requirements have come, such as higher temperatures of use and greater resistance to oxidation. A recent approach has been the development of new materials that have properties intermediate between those of traditional organic systems (i.e. polymers) and those of traditional inorganic systems (i.e. ceramics).
Epoxy networks based on diglycidyl ether of Bisphenol A and poly(oxypropylene)diamine modified with well-defined nanobuilding blocks - polyhedral oligomeric silsesquioxanes (POSS) or in-situ generated silsesquioxane domains - via sol-gel process from alkoxysilanes have been studied. DMA, SAXS, NMR and TEM analysis were used to determine the structure-properties relationship. The comparison and diferences in the structure and thermomechanical properties of these hybrid materials are discussed.
D. GROMADZKIa, P. ČERNOCHa, M. JANATAa, V. KŮDELAa, F. NALLETb, O. DIATc, P. ŠTĚPÁNEKa
a Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, 162 06 Prague, Czech Republic
b Centre de Recherches Paul Pascal, CNRS, 33 600 Pessac, France
c Structures et Propriétés d'Architectures Moléculaires, UMR 5819 (CEA, CNRS, UJF)
DRFMC/SPrAM, CEA Grenoble, 38054 Grenoble Cedex 9, France
The morphological behavior of partially sulfonated polystyrene-block-poly(ethylene-alt-propylene) (PS-PEP) membranes cast from tetrahydrofuran (THF) solutions were investigated by small-angle X-ray scattering (SAXS) and atomic force microscopy (AFM). The uptakes of methanol and water increase as the sulfonation degree increases, the methanol uptake being overwhelmingly greater than the water uptake. The conductivity increases almost exponentially with increasing sulfonation degree of polystyrene units. Clusters of sulfonated units that are formed in the solution used for casting membranes persist in the solid state after evaporation. In contact with water, swelling of the membranes proceeds predominantly in these clusters. The original lamellar morphology of the diblock copolymer is progressively deformed with increasing degree of sulfonation by the presence of the clusters containing ion-rich sequences of sulfonated polystyrene blocks.
We acknowledge support by the Grant Agency of the Czech Republic (SON/03/E001) within the EUROCORES Programme SONS of the European Science Foundation, which is also supported by the European Commission, Sixth Framework Programme
Z. TUZARa, P. ŠTĚPÁNEKa, V. RYUKHTINb, P. KADLECa, F. NALLETc, L.NOIREZd 1
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic (tuzar/imc.cas.cz)
bNuclear Physics Institute, Academy of Sciences of the Czech Republic, 25 068 Rez near Prague, Czech Republic
cCentre de Recherches Paul Pascal, 115 Av. A. Schweitzer, 33 600 Pessac, France (nallet/crpp-bordeaux.cnrs.fr)
dLaboratoire Leon-Brillouin CEA-Saclay, 91191 Gif-surYvette Cedex, France
Mixtures of cyclohexane (CX) and dimethylformamide (DMF) form one-phase or two-phase systems, depending on temperature and composition. The borderline between the two regimes is called the coexistence curve or the binodal. In presence of a diblock copolymer, e.g. polystyrene-polyisoprene or polystyrene-poly(ethylene propylene), where DMF is a selectively good solvent for the first block and CX for the second, solutions fail to separate macroscopically. Instead, the system is macroscopically homogeneous and solvents are microphase-segregated to DMF-rich and CX-rich domains, separated by interfaces covered with a diblock copolymer.
Various temperature-dependent dynamic modes of the physical polymer network belonging to the cooperative diffusion, selfdifusion of the copolymer molecules, and large-scale heterogeneities can be observed by dynamic light scattering. The structural features become regular at temperatures well below the coexistence curve of the solvents and give rise to characteristic patterns in small angle neutron scattering. This method shows that the low-temperature ordered phases have in all cases examined a hexagonal or cubic symmetry. Typical diameter of the structure building blocks (cylinders or spheres) is 15 nm, their typical distance is 80 nm. Macroscopic morphology (large grains size of several mm) can be observed by ultra small-angle neutron scattering.
We acknowledge support by the Grant Agency of the Academy of Sciences of the Czech Republic (A4050403).
Y.A. Shchipunov
Institute of Chemistry, Far East Department, Russian Academy of Sciences
690022 Vladivostok, Russia, YAS/ich.dvo.ru
Polysaccharides and proteins were used to fabricate hybrid biopolymer-silica nanocomposites by the sol-gel technique. As a silica precursor, tetrakis(2-hydroxyethyl) orthosilicate was used. It possesses a complete compatibility with biopolymers that was obvious from the absence of denaturating effect and precipitation or phase separation in the system after its mixing with biopolymer in a solution. This allowed synthesizing monolithic hybrid nanocomposites with most of practically important polysaccharides and such proteins as albumin, gelatin and casein.
All the studied biopolymers played an important role in the sol-gel processes, catalyzing them. By this reason, they took place at any pH value and decreased temperature.
The sol-gel processing leads to a formation of hydrogel. It was demonstrated that this can be applied for jellifying solutions of polysaccharides that do not form gels. Hydrogels were used further to fabricate xerogels and aerogels. The properties and structure of all the types of prepared nanocomposites materials were studied with the help of a dynamic rheology, differential scanning calorimetry, scanning electron microscopy and atomic force microscopy.
It was found that the biopolymers served as a template for silica generated in situ by the sol-gel processing. Biopolymer macromolecules were covered by an inorganic shell owing to silica nucleation and subsequent growth of precipitate. This was demonstrated by the atomic force microscopy. The suggested mechanism differs from that for the sol-gel processing performed with common silica precursors. The silica precipitation on biopolymer macromolecules determined the structure and properties of hybrid nanocomposites. They depended on the biopolymer type, charge and concentration as well as conformation of their macromolecules. Although the macromolecules were covered by silica shell, biopolymers did not loose their ability for conformational rearrangements. It was demonstrated in a case of hybrid nanocomposites materials with entrapped kappa- and iota-carrageenans and gelatin. These polysaccharides and protein experienced a reversible coil-to-helix transition with varying the temperature that provided a thermoreversible change in the mechanical properties of hybrid materials around the biopolymer phase transition temperature.
Clément HOUGA, Jean François LE MEINS[1], Daniel TATON, Yves GNANOU and Redouane BORSALI
Laboratoire de Chimie des Polymères Organiques, ENSCPB,
16 avenue Pey Berland, 33607 Pessac.
The aim of this study is to exploit intrinsic properties of polysaccharides (Complexation with divalent cations, gelation, specific transition with temperature …) in a self assembled form obtained via a copolymer. We have decided to study linear block copolymer based on polysaccharides which have received few attention up to now. We have elaborated in a first attempt, dextran-b-polystyrene, to validate a novel chemical methodology which consists in using the Dextran (![]() ~6000g/mol) as a macroinitiator in an atom transfer radical polymerisation process (ATRP). The chemical procedure is indicated in Scheme1.
~6000g/mol) as a macroinitiator in an atom transfer radical polymerisation process (ATRP). The chemical procedure is indicated in Scheme1.
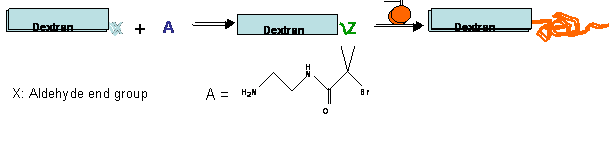
Scheme 1.
We exploit the isomer containing the aldehyde end group to end functionalize the dextran by reductive amination. Several block copolymers with different PS length, characterized by size exclusion chromatography, Infrared spectroscopy and 1HNMR present different solubility properties. Experiments based on Atomic Force Microscopy and dynamic light scattering experiments prove their ability to self assemble in solution. A deep study of the assembly process and optimisation of the chemical synthesis before extending to other polymers are in progress.