1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
CHARACTERIZATION OF AGED AND PROCESSED PP BY IMAGING CHEMILUMINESCENCE
M. HAMSKOG, P. GIJSMAN, B. TERSELIUS
Kristianstad University, School of Engineering, Sweden
Plaques of polypropylene (PP) were artificially recycled using repeated cycles of extrusion and oven ageing. The oxidation induction time (OIT) and total luminescence intensity (TLI) were determined using a newly developed mutli-cell imaging chemiluminescence instrument.
Complementary characterization was performed using microcalorimetry, FTIR, yellowness index and tensile testing. It was observed that the addition of 20 % of degraded PP to virgin PP caused deterioration of properties. The mixtures showed clear evidence of heterogeneous degradation. Addition of extra stabilizer increased OIT.
CHEMILUMINESCENCE IS SHEDDING LIGHT ON DEGRADATION AND STABILIZATION OF PLASTICIZED POLY(VINYL)CHLORIDE.
Y. KANNa,b, N. C. BILLINGHAMb
a Lynn Plastics Co. LLC, 92 Brookline St, Lynn, MA 01902, USA
bDepartment of Chemistry, University of Sussex, Brighton, BN1 9QJ, UK
Aspects of thermo- and photo-degradation and stabilization of flexible PVC compounds have been studied with the aid of chemiluminescence. The intensity of the chemiluminescence (CL), emitted during the degradation of PVC is shown to be proportional to the concentration of the polyene structures and to the peroxy radicals, formed upon their oxidation.
A discussion of thermal stabilization of flexible PVC compounds is focused on the action of Ba/Zn carboxylates. It has been shown that Ba/Zn carboxylates, yielding good initial or short-term color stability at high temperatures or good low/moderate temperature stability perform by blocking of the propagating polyenes. The long-term stability of compounds stabilized with this type of stabilizers is usually poor, because polyenes, created by acid-catalyzed thermolysis of labile esters, forming during replacement of labile chlorines by carboxylates, have significantly lower stability and contribute to fast degradation. For the long-term stability, the ability of the stabilizer to neutralize hydrogen chloride, evolved by dehydrochlorination is more important than the ability to block polyenes.
By knowing the chemistry of mixed metal carboxylates, i.e. metal ratio, type of carboxylate, short- or long-term stability (or both) can be improved. Monitoring CL is an excellent tool in this respect, because it can detect polyene formation and differentiate functionalities of different stabilizers in terms of their ”blocking” abilities.
The applicability of the CL technique in assisting with additive selection (e.g. plasticizers, HCl absorbers, phosphites, antioxidants) for providing formulated compounds with the required heat stability is demonstrated.
The usefulness of the CL analysis is also shown in building correlations with long-term weathering tests and thus minimizing time for development of formulations. Unlike other accelerated weathering tests (e.g. EMMAQUA and Xenon), typically not completely accurate for prediction of PVC photo-stability, the CL technique gives correct assessment of the UV stability of designed materials.
THERMAL OXIDATION OF CELLULOSE INVESTIGATED BY CHEMILUMINESCENCE; EXTRAPOLATION OF STABILITY DATA TO CONDITIONS CLOSE TO AMBIENT
J. RYCHLÝ, L.MATISOVÁ-RYCHLÁ, *, I. LACÍK, K. SLOVÁK,
Polymer Institute*, Centre of Excellence for degradation of biopolymers,
Slovak Academy of Sciences, Dubravská c.9, 842 36 Bratislava, Slovak Republic
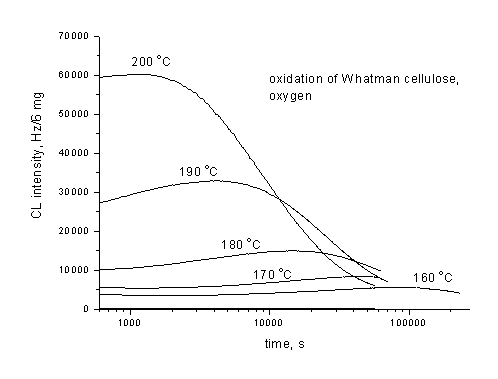
An attempt to estimate the degradation of cellulose, and of some other glucans from chemiluminescence measurements performed in oxygen and in nitrogen has been made. The relatively good coincidence between rate constants of the first order determined from chemiluminescence non-isothermal experiments for cellulose in oxygen and those from polymerization degree decay [1] is a good pre-requisite for generalization of the above approach to other materials of the similar structure. However, there are still many questions, which await for the proper interpretation. The present paper quotes a few of them:
Up to now knowledge on the subject and demonstration of the potential and limitations of the chemiluminescence method in the research of degradation of polysaccharide based materials has been outlined. Experiments were performed on the circular sheets of the material or on powder of the mass not exceeding 5 mg. Impregnation with additives has been done with 20 mg solution of an additive in 5 ml of distilled water and the sample was dried overnight at room temperature and protected from the day light.
Acknowledgments
The authors gratefully acknowledge the support of the European Community, 5th Framework Energy, Environment and Sustainable Development programme, contract no. EVK4-CT-2000-00038 (PAPYLUM). The work is the sole responsibility of the authors and does not represent the opinion of the Community. The Community is not responsible for any use that might be made of the data appearing herein.
[1] J.Rychlý, M. Strlič, L.Matisová-Rychlá, J.Kolar, Polym.Degrad.Stab. 78, 357 (2002)
Corresponding author: Tel.: 00421 2 5477 1626, Fax: 00421 2 5477, E-mail: upoljory savba.sk

Fig. 1. Isothermal chemiluminescence runs of oxidation of Whatman cellulose in oxygen
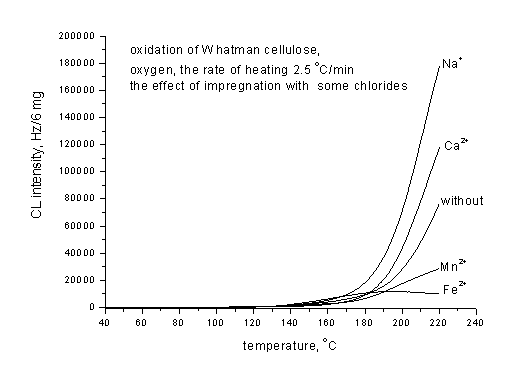
Fig.2. Non-isothermal chemiluminescence at the oxidation of Whatman cellulose impregnated with different chlorides. The rate of heating 2.5 oC/min
THE ROLE OF WEAK SITES IN THE LONG TERM AGING OF HDPE UNDER THE EFFECT OF EXTRACTIVE MEDIA
C. KISS1,2, E. EPACHER1,2, P. STANIEK3, B. PUKÁNSZKY1,2
1 Budapest University of Technology and Economics, Department of Plastics and Rubber Technology, H-1521 Budapest, P.O. Box 91, Hungary
2 Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, H-1525 Budapest, P.O. Box 17, Hungary
3 Clariant Huningue, Business Line Plastics Additives, F-68331 Huningue Cedex, BP 149, France
Polyethylenes produced in any polymerization process contain various amounts of functional groups different from the regular - CH2- units. During the high temperature processing of polyethylene as well as during the application of the product, these groups act as weak sites and take part in numerous degradation reactions. As a result of these reactions, chain scission and the increase of molecular weight may occur simultaneously, which lead to the modification of the chemical structure of the polymer chains and the macroscopic properties of the polymer. Unsaturated (vinyl, vinylidene, vinylene) groups, chain branches and oxygen containing functional groups (carbonyl, peroxide, carboxyl, etc.) are the most frequent chain irregularities, which may be regarded as weak sites in polyethylenes. Although the existence of weak sites is an accepted fact and the deleterious effect of vinyl groups on the processing stability of PE has been proven several times [1-3], the relative importance of the various sites, as well as the quantitative correlation between their concentration and the changes in properties are not known yet. The goal of this study was to determine the effect of processing history on the structure of a Phillips type HDPE and on its behavior during long-term storage in water.
A Phillis type polyethylene was extruded six times and the processed polymer was characterized by various methods. Compression molded plates were produced from the samples and stored in water for a year. Specimens were withdrawn from the vessels in regular intervals and their color, MFI and functional group content was determined as a function of soaking time. Surprisingly, the polymer subjected to a single processing step was more sensitive to degradation than those extruded several times. The results indicate that severe oxidation takes place in this sample, which leads to chains scission and to the change of color. Mechanical properties deteriorated rapidly, but improved again at longer storage times. The chemical composition and properties of samples extruded several times changed much less as a function of storage time. We explained the differences with the consumption of weak sites during processing and with consecutive reactions during storage.
1. Moss, S., Zweifel, H., Polym. Degr. Stab. 25 , 217 (1989)
2. Drake, W.O., Pauquet, J.R., Todesco, R.V., Zweifel, H., Angew. Makromol. Chem. 176/177 , 215 (1990)
3. Hinsken, H., Moss, S., Pauquet, J.R., Zweifel, H., Polym. Degr. Stab. 34, 279 (1991)
Thermal degradation and ozone attack on styrene-ethylene-butylene-styrene (SEBS) copolymer
N.S. Allen1, M. Edge1, D. Mourelatou1, A.W. Wilkinson1, C.M. Liauw1, M.D. Parellada2, J.A. Barrio2, V. Ruiz Santa Quiteria2
1Centre for Materials Science Research, Department of Chemistry and Materials, Faculty of Science and Engineering, The Manchester Metropolitan University, Chester Street Manchester Ml 5GD, U.K.,
2Repsol – YPF, S.A., Centro Tecnológico, C/Embajadores, 183, 28045 Madrid, Spain
The thermal and ozone degradation of hydrogenated poly[Styrene-b-butadiene-b-styrene] or poly[styrene-b-(ethylene-co-butylene)-b-styrene], (SEBS) has been studied using a variety of analytical and spectroscopic methods including thermal analysis, UV, luminescence and FTIR spectroscopy coupled with crosslinking and hydroperoxide analysis in order to commercial unstabilised material results in chain scission and severe crosslinking giving rise to extensive discolouration. FTIR analysis shows complex degradation.processes with distinct features associated with each phase. There is a solvent soluble clear phase showing oxidation due primarily to the aliphatic part with a predominant absorption associated with terminal carboxylic acid groups at 1713 cm-1. There is also a solvent insoluble phase, which is predominantly crosslinked aliphatic material due to the formation of hydroperoxides and peracids/peresters. Vinyl groups are also evident in this phase. Thus, end group oxidation is a predominant process with the immediate autocatalytic formation of high concentrations of primary hydroperoxides during the early stages of oxidation. Phosphorescence analysis also indicated the presence of initial acetophenone chromophores, which are associated with polystyrene end groups formed by chain breakage at the aliphatic links. These species can act as initial active sensitive sites for further breakdown, possibly via a thermally induced hydrogen atom abstraction process. The end-chain aliphatic radicals are the sites for initial rapid hydroperoxidation. Mechanisms are proposed and discussed for each phase oxidation. With ozone exposure the original functionalities in the SEBS based on aliphatic vinyl and aromatic (styrene) structures were observed to decrease in intensity and these were consistent with the concurrent formation of ozonide groups. Immediate exposure of SEBS to ozone resulted in the rapid and consistent formation of a variety of carbonyl and unsaturated carbonyl products based on aliphatic esters, ketones, and lactones as well as aromatic carbonyl associated with the styrene phase. These were followed by a more gradual formation of ether, hydroxyl and terminal vinyl groups with time and concentration. Unlike thermal oxidation whist there was strong evidence for hydroxyl group formation hydroperoxide analysis showed minimal evidence for active peroxides although growth was consistent with ozone dosage. No crosslinking was also found in this treated material. Early decreases in in-chain vinyl groups by FTIR analysis were also consistent with an observed decrease in fluorescence functionalities in the SEBS associated with primarily trans-stilbene groups whereas longer periods of exposure showed new fluorescence functionalities. Phosphorescence analysis showed the formation of acetophenone end groups on the styrene chains associated with chain scission within the aliphatic rubber-styrene interphase region. Commercially ozone resistant SEBS materials were found to contain lower levels of fluorescent trans-stilbenic chromophores indicating this to be the weak link at the interphase. Mechanistic routes for these processes are also proposed and discussed.
HYDROTHERMAL STABILITY OF GLASS FIBER REINFORCED POLYETHYLENE TEREPHTHALATE COMPOSITES : A MULTI-SCALE APPROACH
M.P. FOULCa, A. BERGERETa, L. FERRYa, P. IENNYa, A. CRESPYb
aEcole des Mines d’Alès, CMGD, 6 avenue de Clavières, 30319 Alès Cedex, France
bUniversité de Toulon et du Var, R118, BP 132, 83957 La Garde Cedex, France
Glass fiber reinforced polyethylene terephthalate (PET) composites can be considered as accurate materials that can be used to replace die cast metals and for under-the-hood pieces, so that resistance to hot hydrolysis is a key requirement for these materials. A previous study [M.P. Foulc, A. Bergeret, L. Ferry, P. Ienny et A. Crespy, 2nd International Conference on Polymer Modification, Degradation and Stabilisation, Budapest, Hungary, 2002] was carried out on a commercial composite system (Rynite 530, DuPont de Nemours Co.) aged at 120°C/1.6 bars/200h. Two main degradation phenomena were identified and discussed : an inherent fragility of the polyester matrix (decrease in the molecular weight, increase in the number of acidic end groups, increase in the water absorption rate inducing a plasticization effect) and a degradation of the fiber/matrix adhesion (formation of cracks). To complete the understanding of the degradation, the present work consists in the study of the influence of the fiber content (20 to 45 wt%) and of the fiber surface treatment (more or less compatible with the polyester matrix) on the composite stability with regard to hot water. Emphasis has been put on an approach conducted at the following different scales :
ARTIFICIAL AND NATURAL WEATHERING OF PP-PALM FIBER COMPOSITES: MECHANICAL AND SPECTROSCOPIC CHARACTERIZATION
B. ABU-SHARKH
Department of Chemical Engineering, KFUP, Dhahran, 31261, Saudi Arabia
Polypropylene composites containing palm cellulose fibers were weathered in an artificial environment using the Xenotest Beta-LM weatherometer. The samples were also exposed for 12 months in the severe weather conditions of the Eastern province of Saudi Arabia where summer temperatures can exceed 50oC. The composites showed great stability compared to pure polypropylene and the mechanical strength of the artificially weathered samples declined by a maximum of 15 % after exposure for 2000 hours. On the other hand, strength of pure polypropylene declined by as much as 80%. Weathered samples were also characterized by GPC and FTIR to investigate change in molecular weight distribution and formation of new functionalities as a function of exposure. Natural weathering yielded similar information and the composite material was much more stable than the pure polypropylene. The stability of the composite materials can be attributed to presence of natural antioxidants in the cellulose fibers that scavenged the free radical products of UV irradiation, thus preventing further degradation of the polymer.
Bisphenol A Poly(carbonate) recycling : rheological & calorimetric study
J. F. Feller*, A. Bourmaud, Y. Grohens
Polymers & Processes Laboratory, South Brittany-University, 56 321 Lorient, France
In the last decades, a great deal of attention has been focused on plastics recycling. Due to an increase of consideration of human activity impact on environment, it is meaningful to introduce recycling in the products design. Several ways to use alternative feedstock for production of plastics are known, resulting from thermal, mechanical, chemical or enzymatic recycling. Mechanical recycling of post consumer commodity products and engineering plastics wastes can provide an interesting feedstock if a few contaminant are introduced and that degradation due to successive processing is properly characterized.
In the present study, we have investigated the evolution of
rheological and calorimetrical properties of bisphenol A
poly(carbonate) (PC) wastes after each grinding/injection cycle
and this for eight cycles. Cone/plate rheometry (CPR) shows that
the shift of ![]() curves
to lower viscosities is particularly observed at 240°C, from the
first to the fourth cycle. Moreover, the G’/G’’ cross over
frequency increases with the number of cycles. From Dynamical
Mechanical Analysis (DMA) it can be noticed that the glass
transition temperature Tg decreases and the storage
modulus E’ at 30°C increases after four cycles. Tg
decrease is confirmed by DSC measurements together with an
increase of Cp.
Accelerated aging experiments in U.V. irradiation have also been
proceeded and show an important color change after fifteen day of
exposure.
curves
to lower viscosities is particularly observed at 240°C, from the
first to the fourth cycle. Moreover, the G’/G’’ cross over
frequency increases with the number of cycles. From Dynamical
Mechanical Analysis (DMA) it can be noticed that the glass
transition temperature Tg decreases and the storage
modulus E’ at 30°C increases after four cycles. Tg
decrease is confirmed by DSC measurements together with an
increase of Cp.
Accelerated aging experiments in U.V. irradiation have also been
proceeded and show an important color change after fifteen day of
exposure.
The results show that the effect of recycling, certainly resulting in molecular weight decrease is more sensitive on viscosity than on modulus.
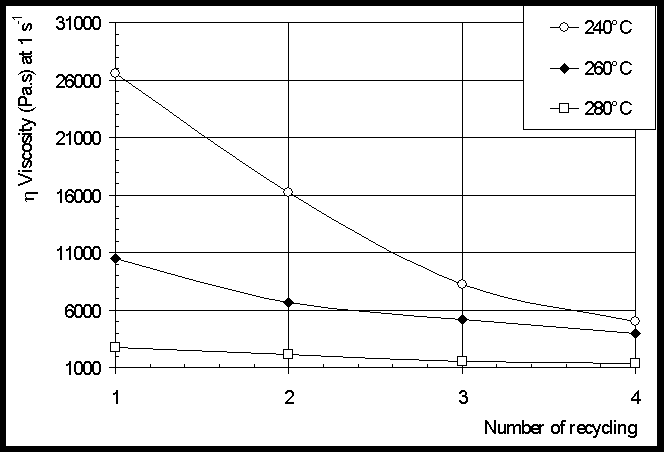
POST-CONSUMER POLYOLEFINS AND CONDENSATION POLYMERS RECYCLING BY REACTIVE BLENDING
M. AGLIETTO, M.-B. COLTELLI, S. SAVI
Dipartimento di Chimica e Chimica Industriale, Università di Pisa
Via Risorgimento 35 – 56126 Pisa, Italy; e-mail:aglaim dcci.unipi.it
Blends of polyolefins (PO) and condensation polymers are of large interest as new materials with modulated properties depending on the composition and the phases dispersion. For example the blending of polyethylenterephthalate (PET) or nylon 6 [polyamide 6(PA6)] with polyethylene by melt mixing, could enhance the Young Modulus and the barrier properties towards O2 and CO2 (1). Moreover the use of plastics coming from the separate collection of solid waste to obtain blends provides a suitable way for waste recycling (2) obtaining a material with an added value in respect to the starting polymers.
Condensation polymers and polyolefins are thermodynamically immiscible, so the uncompatibilized blends exhibit a coarse morphology that corresponds to very poor mechanical properties. Satisfactory performances in immiscible blends is usually attained by minimizing interfacial tension and improving adhesion between the two phases. One of the most studied method to enhance compatibility consists of adding to the immiscible system a polyolefin with grafted reactive groups that can react in the melt with either hydroxyl (PET) or amino (PA6) terminal groups, thus yielding comb copolymers (3) able to stabilize the interfaces between the two immiscible phases providing the requested adhesion for properties modulation.
The efficiency of the above reactions at a molecular level depends on different parameters, such as the structure of the condensation polymer and polyolefin, the type and structure of interfacially reactive pre-compatibilizer, the presence and type of catalyst, the original phases segregation, the formation in situ of reactive species and the operative conditions.
These parameters are analyzed in details with special reference to the use of recovered polymers (polyolefin and condensation polymer),which can also contain reactive species formed during the use.
The results are then discussed in terms of possibility to drive these type of processes to useful recycled materials.
References
NEW DEVELOPMENTS IN PROCESSING STABILIZATION OF POLYOLEFINS
CH. KRÖHNKE, P. STANIEK, J. MALÍK
Clariant Huningue, BP 149, F-68331 Huningue, France
Stability and usability of thermoplastic articles such, as those made from polyolefins are strongly dependent on the conditions during their processing. Usually polymers are exposed during this processing step to mechanical stress created by high shear forces in the extruder as well as by a variety of thermally induced chemical reactions affecting the polymer backbone.
As discussed since long time polymer degradation is believed to occur by radical processes under formation of carbon and mainly oxygen centered radicals. At high processing temperatures, the most aggressive species are hydroperoxide radicals.
Generally used are sterically hindered phenolic compounds, which repress certain degradation, but causing often (to a different extent) a discoloration of stabilized polymer resin by formation of colored products with quinoid structures. It is further known from the literature that combinations of those phenolic compounds with organo-phosphites or -phosphonites yield synergistic effects both towards improved melt flow maintenance and clear improvement in color stabilization.
Many of these phosphites and phosphonites have some inherent drawbacks as being principally hydrolysable or, in some cases, are insufficiently compatible to the polymer matrices leading to undesired effects like blooming or loss of the stabilizers.
Possibilities to overcome these and other disadvantages are given by organo-based phosphines, which can be used as highly efficient processing stabilizers. A tailor-made molecular structure leads to a novel developmental product, offering a unique efficiency already at very low concentrations in all types of polyolefins. In addition, the physical and chemical properties such as high polymer compatibility, very low volatility and inherent hydrolytic stability offer additional benefits for the new product – as compared to classical stabilizer types.
OXIDATIVE CROSSLINKING DURING POLYMER PROCESSING. APPLICATION TO POLY(ETHYLENE TEREPHTHALATE) RECYCLING
X. COLIN, R. ASSADI, J. VERDU
Laboratoire de Transformation et de Vieillissement des Polymères, Ecole Nationale Supérieure des Arts et Métiers, 151 Boulevard de l‘Hôpital, 75013 Paris, France
E-mail: LTVP paris.ensam.fr
Some polymers undergo non-reversible structural changes during their processing despite the fact that they are thermally stable at the temperatures under consideration. One possible cause for these modifications is oxidation: when oxygen is in low concentration, which is generally the case during polymer processing, a predominant crosslinking can occur by coupling of alkyl radicals and thus, a catastrophic gelation can occur at long term.
A kinetic model capable to predict this behaviour and its consequences in rheological properties is proposed. It starts from the standard kinetic scheme for radical chain oxidation, initiated by unimolecular hydroperoxide decomposition, and links molar mass to melt viscosity using the classical Bueche’s law. Theoretical results are compared to experimental data relative to PET recycling by extrusion. These results reveal the good predictive qualities of the model.
EFFICIENT METHOD OF MATERIAL RECYCLING OF MUNICIPAL PLASTIC WASTE
I. FORTELNÝ, D. MICHÁLKOVÁ, Z. KRULIŠ
Institute of Macromolecular Chemistry of the Academy of Sciences of the Czech Republic, Heyrovsky Sq. 2, 162 06 Prague 6, Czech Republic
The municipal plastic waste, after manual separation of PET bottles, is a mixture of various grades of polyethylene, polypropylene and polystyrene with small amounts of other plastics (PET, polyamide, etc.). It was found that a mixture of ethylene-propylene elastomers (EPR) with styrene-butadiene block copolymers (SB) is a very efficient compatibilizer for PE/PP/PS blends [1]. For blends containing a degraded polyolefin, a strong synergy between the effects of the compatibilizer and a secondary-amine-based stabilizer on impact strength of the blends was observed. Using this knowledge, a very efficient compatibilization procedure for municipal plastic waste was developed [2]. Application of this procedure using 5 % of EPR-SB compatibilizer leads to recycled materials with mechanical properties comparable with virgin polyolefins. Therefore, these results can be used not only for significant improvement of mechanical properties of articles from recycled materials but also for development of recycled materials which can substitute virgin polymers, in particular polyolefins, in many applications such as geosynthetic elements, transport pallets, water tubes.
Acknowledgement
The authors are grateful for support to the Academy of Sciences of the Czech Republic (project S4050008)
References:
1. Z. Kruliš, Z. Horák, I. Fortelný, D. Michálková, Czech Patent No. 290957
2. Z. Kruliš, D. Michálková, I. Fortelný, Z. Horák, Czech Patent Application PV 2002 - 3589
Degradation of vulcanized rubbers AND POLYURETHANES by biomimetic free radical reactions catalyzed by oxidative enzymes
T. WATANABEa, S. SATOa, Y. Ohashia, Y. HONDAa, K. Muraokab, T. KIMURAc
a Laboratory of Biomass Conversion, Wood Research Institute, Kyoto University, Gokasho Uji Kyoto 611-0011, Japan, e-mail: twatanab kuwri.kyoto-u.ac.jp.
b Sumitomo Rubber Industries, Ltd. Kobe 651-0071, Japan
c Bio Research Laboratory, Future Project Division, Toyota Motor Corporation. Toyota, Aichi 471-8572, Japan
Considerable attention has been paid to the problem of disposing of or recycling polymers. With regard to rubbers, extensive studies have been conducted into decomposing rubber products by microorganisms or enzymes. However, it was an open question whether rubbers can be decomposed by the action of one specific enzyme. We herein report that rubbers were degraded by lipid peroxidation catalyzed by a purified oxidative enzyme. Lipid peroxidation is an extracellular free radical reaction catalyzed by selective lignin-degrading fungi such as Ceriporiopsis subvermispora. This fungus produces unsaturated fatty acids and oxidize them with manganese peroxidase (MnP) to generate lipid-derived free radicals1). The systems have been proposed as a reaction decomposing lignin at a site far from enzymes. We applied the lipid peroxidation to the rubber docomposition. Vulcanized polyisoprene rubbers were decomposed by the free radicals from linoleic acid (LA) produced by MnP at 35 °C for 96 h.
Polyurethanes are an important and versatile class of man-made polymers. Polyurethanes are classified into two types, polyester and polyether polyurethanes. Enzymatic attack on polyester polyurethanes could be contributed by hydrolases such as ureases, proteases and esterases. However, little is known about the enzymatic degradation of polyether polyuretanes. Using lipid peroxidation catalyzed by laccase, the polyether polyurethanes were almost completely degraded for 2 weeks. Thus, lipid peroxidation controlled by the oxidative enzymes give us a new strategy for the safe disposal and recycling of polymers.
1) T. Watanabe, S. Katayama, M. Enoki, Y. Honda, M. Kuwahara: Eur. J. Biochem., 267, 4222 (2000).
2) S. Sato, Y. Honda, M. Kuwahara, T. Watanabe: Biomacromolecules, 4, 321 (2003).
CONTRIBUTION TO THE CHEMICAL RECYCLING OF PET BOTTLES
S. FELLAHIa *, Z. SAFFIDINEb
aPolymer Engineering Department, Sonatrach – Institut Algérien du Pétrole ; Boumerdes, 35000 ALGERIA
bEMP, Algiers, Algeria
*Corresponding author
In this work, soft drink PET bottles have been treated with an excess of glycol’s (Ethylene glycol, di-ethylene glycol and propylene glycol) leading to a trans-esterification reaction. The reduction of higher molecular weight PET to short chain fragments is achieved by heating the PET in the presence of a glycol (di-ethylene glycol) and a catalyst. During this glycolysis reaction, some of the free DEG replaces the EG in the polyester chain by a process of chain scission and glycol exchange. The resulting major products are called polyols. Typically, this glycolysis reaction takes place over 04 hrs duration at 220°C (reflux temperature) with a 3/1 DEG/PET ratio. The reaction was carried out under a continuous nitrogen purge to inhibit degradation of the resulting polyols. These polyols were used to prepare PUR foams.
The characterization of these polyols was done using Infrared spectroscopy, chromatography, total basic number, density and kinematic viscosity.
MELT REACTION IN POLYMER BLENDS COMPRISING PHBV AND ENR
C.H. CHANa, J. ISMAILa, H. W. KAMMERb
aSchool of Chemical Sciences, University Sains Malaysia, Malaysia
bUniversity of Halle, Germany
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) with 12 mole % of hydroxyvalerate content is a natural aliphatic polyester. At temperatures above its melting point, thermal decomposition occurs leading to shorter chains with carboxyl end groups. In blends with epoxidized natural rubber (ENR), 50% epoxidation level, these carboxyl chain ends may trigger reactions between the constituents since the epoxidized groups provide reactive sites. Morphological studies reveal immiscibility of the polymers over the entire composition range. Hence, any reaction between the constituents is restricted to the interfacial region between the components. We report here on studies on melt reactions between the components at elevated temperatures. DSC traces display an exothermic peak when the blends are annealed at temperatures ranging from 220 up to 234 °C. The kinetics of the reaction is discussed in terms of a serial reaction scheme. It turns out that the reaction rate increases with annealing temperature Ta. Morphological studies by optical microscopy reveal that PHBV is still capable of crystallizing after blends were subjected to the annealing procedure. Either ring-banded or fibrillar spherulites can be seen in blends with PHBV in excess. We note, however, that the rate of crystallization is dramatically reduced after annealing at temperatures in the indicated range.
ENHANCING LIFETIME OF GLAZING APPLICATIONS BY TAILOR-MADE, IMPROVED STABILIZER PACKAGES
M.C. GROB
Ciba Specialty Chemicals Inc., Plastic Additives, Head of Skill Center Extrusion, CH-4002 Basel, Switzerland
Glazing applications made out of plastics are designed to withstand several years of outdoor exposure in a variety of climates, in fact end-user expectations for the stability of such applications are usually beyond 10 years. In order to resist severe outdoor conditions and reaching long lifetimes, an excellent protection is required for processing as well as long-term stability. Only high performance light stabilizer systems allow for long lifetimes under retention of materiel properties. Most prominent glazing polymers – polycarbonate, polyesters, PMMA – can be stabilized in different ways:
For polycarbonate and polyester sheets two main techniques are established, coating with a UV protective finish or coextrusion with a thin cap layer made of polycarbonate or polyester containing a high UV absorber concentration. For very demanding applications high performance UV absorbers based on hydroxyphenyltriazine open the door for especially long life times, which were not achievable with the classic benzotriazole based UV absorbers. This is possible thanks to the enhanced inherent stability of the triazine based UV absorbers and to their very strong absorption in the UVB range, where these two polymer classes show their highest sensitivity.
PMMA is, thanks to its low absorption, inherently quite stable for outdoor use. Long lasting applications however are usually stabilized with a combination of hindered amine stabilizers and UV absorbers. Depending on the thickness, the system has to be adapted to the final application. In colored applications the best performing system is strongly depending on the colorant and can vary from case to case. Some dyes cannot be stabilized for outdoor applications due to their sensitivity outside of the UV range.
Besides the protection from degradation by light, these polymeric glazing materials can receive further enhanced properties by the use of specialized, functional additives: Glazing applications can be negatively influenced in their functionality by microorganisms like algae or biofilm. Special ranges of effect additives can prevent the formation of these disturbing nuisances. By the addition of a soluble NIR absorber, the main properties of the glazing material – the transparency - can be retained and the heat transfer through the polymer can be directly influenced. This opens new opportunities to replace glass by plastic materials.
PHOTOSTABILIZING ACTION OF A POLYMERIC HALS IN POLYPROPYLENE STUDIED BY REACTIVE THERMAL DESORPTION-GC AND MALDI-MS
H. OHTANIA, Y. TAGUCHIA, Y. ISHIDAB, S. TSUGEA, H. MATSUBARAC
aDepartment of Applied Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan
bResearch Center for Advanced Energy Conversion, Nagoya University, Nagoya 464-8603, Japan
cAichi Industrial Technology Institute, Kariya 448-0003, Japan
Hindered amine light stabilizers (HALSs) are often added to polymeric materials for the prevention of degradation in the outdoor uses. In practical use, polymeric HALSs of which molecular weight ranges from 1,500 to 4,000 are often employed to suppress their bleeding-out from the substrate materials. Generally, it is known that the photodegradation of a given polymeric material notably proceeds when the stabilizers are lost from the substrates by their decomposition and/or bleeding in practical use. Therefore, it is important to know not only the content of the stabilizers remaining in the material but also their structural changes during the actions of stabilization for the lifetime prediction of the polymeric material. In this work, thermal desorption-gas chromatography (TD-GC) assisted by in-line chemical reaction in the presence of a strong organic alkali, tetramethylammonium hydroxide (TMAH) [(CH3)4NOH] and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) combined with solid sampling technique were complementarily applied to the direct determination of trace amounts of a polymeric HALS, Adekastab LA-68LD (MW = ca.1,900), occluded in polypropylene (PP) without using any troublesome pre-treatment such as time-consuming solvent extraction. Moreover, these methods were applied to follow the structural changes of the HALS added in PP materials during UV exposure to clarify the photostabilization mechanisms caused by the HALS.
oligomeric disulfides as stabilizers for polyolefins with intramolecular synergetic effect
L.YU. SMOLIAKA, N.R. PROKOPCHUKA, YU.P. LOSevb
aBelarusian State Technological University, 13-A Sverdlova str., 220050 Minsk, Republic of Belarus
bDepartment of Chemistry, Belarusian State University, 14 Leningradskaya str., 220050 Minsk, Republic of Belarus
E-mail: L_Smoliak Yahoo.com
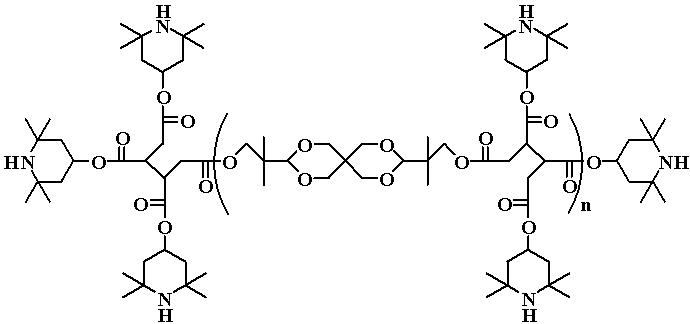
Among the traditional stabilizers for polyolefins there are some classes of chemical compounds: sterically–hindered phenols and amines (primary antioxidants), sulfur- and phosphorus-containing substances. The sulfur-containing substances are recognized as secondary antioxidants or decomposers of hydroperoxides. Ones of tendencies in the development of high performance stabilizers for polymers are the molecular structures or mixtures with different groups, capable to inhibit the oxidative degradation. Such stabilizers can possess a higher performance due to a manifestation of synergetic effect, when two groups operate through different mechanisms.
In our work we have studied the new oligomeric substances of disulfide (DS) class, which contain groups that might give a synergetic effect, in processes of polyolefins (PE and PP) stabilization (See the example of synthesis reaction).
Synthesis of oligomeric DS of biuret
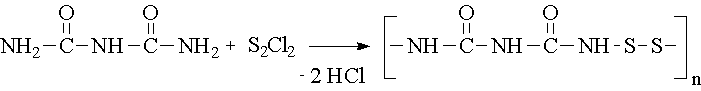
n=8 – 10
More than 10 oligomeric DS substances were synthesized and tested as stabilizers for PE and PP. The dependence of the stabilization efficiency on the DS concentration is investigated and the optimal mole concentration is found. The influence of molecular structure on the stabilization efficiency for the DS is shown and the role of presence such groups as -NH-, –OH, aliphatic chains is discussed. The synergetic action of -S-S- and -NH- groups in one molecule is observed and an original mechanism explaining the high thermostabilization efficiency for some oligomeric DS is proposed.
EFFECT OF ENVIRONMENT AND MICROENVIRONMENT ON ACTIVITY OF POLYMER STABILIZERS
J. POSPÍŠIL, J. PILAŘ, A. MAREK, Z. HORÁK, S. NEŠPŮREK
Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, CZ-162 06 Praha 6, Czech Republic
Information on efficiency of modern stabilizer packages can be obtained in acceptable time by accelerated testing. Attention is paid to understanding of the impact of environmental and microenvironmental factors on stabilizer activity and durability in plastics, rubbers and coatings thus allowing to minimize misleading interpretation of stabilizer mechanisms and polymer lifetime forecast. Selection of realistic testing conditions, knowledge of limits or drawbacks of used methods, interpretation of experimental data and development of selective analytical techniques enhancing understanding of chemical, and physical factors involved in the stabilizing process create a base for a more effective rating and economical use of stabilizer systems. Critical analysis of chemical and physical processes during thermal oxidation and photooxidation reveals a generally heterogeneous character of changes in polymer morphology, formation of concentration gradients of oxygenated products in degrading polymers and in chemical and photochemical consumption of stabilizers, spatial distribution of stabilizer-derived free radical species and migration, partitioning or physical losses of stabilizers affected by testing conditions (temperature, radiation, oxidizing or acid air pollutants). Examples of specific analytical approaches such as electron spin resonance imaging or spectral and chromatographic analyses of microtomed slices of irradiated polymers explaining sacrificial fate of stabilizers are presented.
STABILISATION OF ORTHOPAEDIC UHMWPE WITH VITAMIN E.
L. COSTA, P. BRACCO, E.M. BRACH DEL PREVER, M.P. LUDA
IFM Chemistry Department- University of Torino (Italy)
UHMWPE is widely used for manufacturing hip and knee prosthetic components. According to ASTM F648-98 standard, during the manufacturing of medical grade UHMWPE any stabiliser (i.e. also anti-oxidants) must be avoided. The sterilisation of prosthetic components is performed with high dose of gamma rays or e-beam; recently they are also crosslinked to increase the abrasion resistance by using high energy radiation. However, irradiation with gamma rays or electron beam of these polymeric components can induce its oxidation if the process is carried out in the presence of air. Furthermore, retrieved prosthetic components sterilised with ethylene oxide also show evidence of oxidation. It follows that stabilisation of UHMWPE components would be necessary to avoid degradative oxidation.
In this work we investigate the oxidative stability of UHMWPE additivated with a natural antioxidant, the vitamin E, under e-beam irradiation at different doses.
PHOTOCHEMICAL STABILITY AND PHOTOSTABILIZING EFFICIENCY OF ANTHRACENE/HINDERED AMINE STABILIZERS PROBES IN POLYMER MATRICES
MARTIN DANKO*, ŠTEFAN CHMELA, PAVOL HRDLOVIČ
Polymer Institute, Slovak Academy of Science, 842 36
Bratislava, Dúbravská cesta 9, Slovak Republic, tel.: +421 2
5477 3448, fax: +421 2 5477 5923,
e-mail: upolhrdl savba.sk
Adducts of chromophore-anthracene substituted in position 9- and hindered amine stabilizers (HAS) such as 2,2,6,6-tetramethylpiperidine-4-yl 3-(9-anthracene)propanoate, 1-oxo-2,2,6,6-tetrametylpiperidine-4-yl 3-(9-anthracene)propanoate, [2,2,6,6-tetramethyl-piperidine-4-yl 3-(9-anthracene)propanoate]ium chloride as well as model compounds 3-(9-anthracene) propanoic acid and methyl 3-(9-anthracene)propanoate were investigated. The photochemical stability of adducts was determined at photo-decomposition in polystyrene, polymethyl methacrylate, polyvinylchloride, isotactic polypropylene (iPP) and low density polyethylene (LDPE). Their photo-stabilizing efficiency was determined in iPP and in LDPE. The rate of decomposition of adducts at radiation l > 310 nm was very fast in all matrices. Intramolecularly combined chromophore/HAS are more effective stabilizers than 1 : 1 mixture of separated components. The process of anthracene decomposition produces free radicals containing HAS in structure. These radicals are able to graft on polymer chain of iPP and LDPE.